Page 215 - Materials Chemistry, Second Edition
P. 215
202 3 Metals
Table 3.3. General Types and Properties of Stainless Steels
Type Concentration Properties/applications
Martensitic 11–20 wt.% Cr High hardness, magnetic/cutlery, blades, surgical instruments,
0.15–0.75 wt.% C valves, springs
Austenitic 16–26 wt.% Cr High and low temperature resistance, ductility, superior corrosion
35 wt.% Ni resistance/kitchen sinks, ovens, reaction vessels, food processors,
20 wt.% Mn gutters
Ferritic 10.5–30 wt.% Cr Magnetic, inexpensive/automotive exhaust and fuel lines, cooking
<1 wt.% C, N, Ni utensils, bank vaults, washing machines, dishwashers
Duplex 18–26 wt.% Cr Weldable, high tensile strength, Cl ion resistance (acidic
(austenitic– 4–7 wt.% Ni environments)/desalination plants, food pickling plants,
ferritic) 2–3 wt.% Mo petrochemical plants, pulp and paper industries
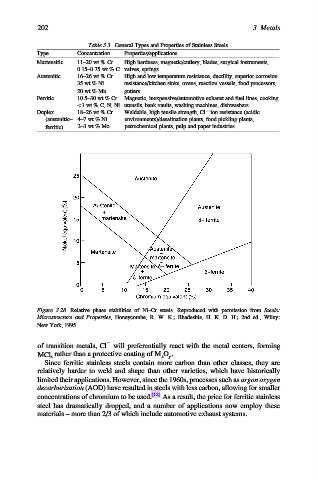
Figure 3.28. Relative phase stabilities of Ni–Cr steels. Reproduced with permission from Steels:
Microstructure and Properties, Honeycombe, R. W. K.; Bhadeshia, H. K. D. H.; 2nd ed., Wiley:
New York, 1995.
of transition metals, Cl will preferentially react with the metal centers, forming
MCl x rather than a protective coating of M x O y .
Since ferritic stainless steels contain more carbon than other classes, they are
relatively harder to weld and shape than other varieties, which have historically
limited their applications. However, since the 1960s, processes such as argon oxygen
decarburization (AOD) have resulted in steels with less carbon, allowing for smaller
concentrations of chromium to be used. [12] As a result, the price for ferritic stainless
steel has dramatically dropped, and a number of applications now employ these
materials – more than 2/3 of which include automotive exhaust systems.