Page 225 - Materials Chemistry, Second Edition
P. 225
212 3 Metals
Fe–C system, with precipitation hardening through dispersion of metal carbides.
Frequently, iron and silicon will be present as processing impurities (or deliberately
added), which will strengthen the alloy due to the formation of Mg 2 Si and Fe 3 Si
precipitates upon cooling. In the presence of Mn, the hardening effect is even more
pronounced, due to the formation of FeMnAl 6 crystallites.
Among the various Al-alloys at our disposal, many of them may not be heat-
treated. In particular, alloys such as pure Al (i.e., containing trace dopants),
Al–Mn, Al–Si, and Al–Mg alloys deleteriously form precipitates along grain bound-
aries. However, Cu–Al and Al–Zn–Mg alloys are greatly strengthened by heat
treatment, through formation of CuAl 2 and MgZn 2 precipitates, respectively. Even
lithium may be added as a hardening agent – forming Al 3 Li precipitates. As with
all age-hardening techniques, the size and dispersion of the crystallites must be
carefully controlled through the heating/cooling regime.
Whereas the solubility of Cu in aluminum metal is ca. 5 wt.% at temperatures in
excess of 500 C, the solubility drops to ca. 0.1 wt.% at room temperature. Hence, a
metastable alloy is present when the high temperature alloy is rapidly quenched.
Subsequent annealing will result in further strengthening similar to what we dis-
cussed for martensite. The strengthening effect is thought to occur due to the
formation of Cu-rich discs (approx. diameter of 100 atoms, and thickness of ca.
4 atoms) that align themselves preferentially with selected planes of the host Al
lattice, causing coherency strains within the solid-state structure.
Refractory metals
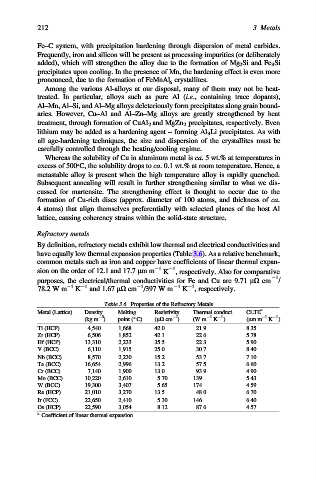
By definition, refractory metals exhibit low thermal and electrical conductivities and
have equally low thermal expansion properties (Table 3.6). As a relative benchmark,
common metals such as iron and copper have coefficients of linear thermal expan-
1
sion on the order of 12.1 and 17.7 mmm 1 K , respectively. Also for comparative
1
purposes, the electrical/thermal conductivities for Fe and Cu are 9.71 mO cm /
1 1 1 1 1
78.2 W m K and 1.67 mO cm /397 W m K , respectively.
Table 3.6. Properties of the Refractory Metals
Metal (Lattice) Density Melting Resistivity Thermal conduct. CLTE a
1
3
1
1
(kg m ) point ( C) (mO cm ) (W m 1 K ) (mmm 1 K )
Ti (HCP) 4,540 1,668 42.0 21.9 8.35
Zr (HCP) 6,506 1,852 42.1 22.6 5.78
Hf (HCP) 13,310 2,233 35.5 22.3 5.90
V (BCC) 6,110 1,915 25.0 30.7 8.40
Nb (BCC) 8,570 2,230 15.2 53.7 7.10
Ta (BCC) 16,654 2,996 13.2 57.5 6.60
Cr (BCC) 7,140 1,900 13.0 93.9 4.90
Mo (BCC) 10,220 2,610 5.70 139 5.43
W (BCC) 19,300 3,407 5.65 174 4.59
Re (HCP) 21,010 3,270 13.5 48.0 6.70
Ir (FCC) 22,650 2,410 5.30 146 6.40
Os (HCP) 22,590 3,054 8.12 87.6 4.57
a
Coefficient of linear thermal expansion.