Page 35 - Materials Chemistry, Second Edition
P. 35
22 2 Solid-State Chemistry
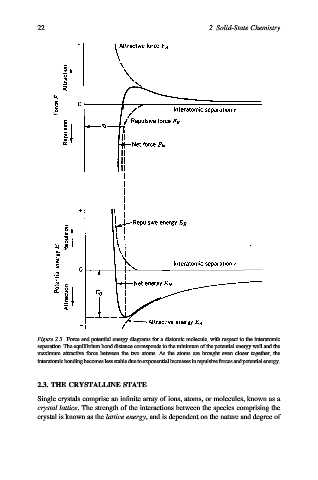
Figure 2.3. Force and potential energy diagrams for a diatomic molecule, with respect to the interatomic
separation. The equilibrium bond distance corresponds to the minimum of the potential energy well and the
maximum attractive force between the two atoms. As the atoms are brought even closer together, the
interatomic bondingbecomes lessstable due toexponentialincreases in repulsive forcesand potential energy.
2.3. THE CRYSTALLINE STATE
Single crystals comprise an infinite array of ions, atoms, or molecules, known as a
crystal lattice. The strength of the interactions between the species comprising the
crystal is known as the lattice energy, and is dependent on the nature and degree of