Page 39 - Materials Chemistry, Second Edition
P. 39
26 2 Solid-State Chemistry
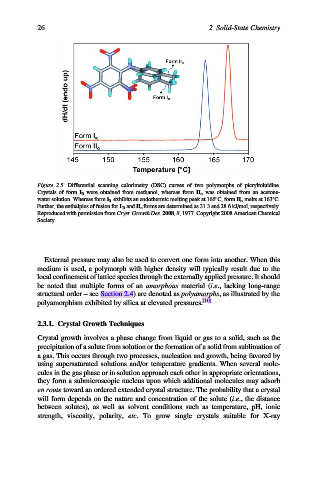
Figure 2.5. Differential scanning calorimetry (DSC) curves of two polymorphs of picryltoluidine.
Crystals of form I R were obtained from methanol, whereas form II o was obtained from an acetone-
water solution. Whereas form I R exhibits an endothermic melting peak at 166 C, form II o melts at 163 C.
Further, the enthalpies of fusion for I R and II o forms are determined as 31.3 and 28.6 kJ/mol, respectively.
Reproduced with permission from Cryst. Growth Des. 2008, 8, 1977. Copyright 2008 American Chemical
Society.
External pressure may also be used to convert one form into another. When this
medium is used, a polymorph with higher density will typically result due to the
local confinement of lattice species through the externally applied pressure. It should
be noted that multiple forms of an amorphous material (i.e., lacking long-range
structural order – see Section 2.4) are denoted as polyamorphs, as illustrated by the
polyamorphism exhibited by silica at elevated pressures. [10]
2.3.1. Crystal Growth Techniques
Crystal growth involves a phase change from liquid or gas to a solid, such as the
precipitation of a solute from solution or the formation of a solid from sublimation of
a gas. This occurs through two processes, nucleation and growth, being favored by
using supersaturated solutions and/or temperature gradients. When several mole-
cules in the gas phase or in solution approach each other in appropriate orientations,
they form a submicroscopic nucleus upon which additional molecules may adsorb
en route toward an ordered extended crystal structure. The probability that a crystal
will form depends on the nature and concentration of the solute (i.e., the distance
between solutes), as well as solvent conditions such as temperature, pH, ionic
strength, viscosity, polarity, etc. To grow single crystals suitable for X-ray