Page 41 - Materials Chemistry, Second Edition
P. 41
28 2 Solid-State Chemistry
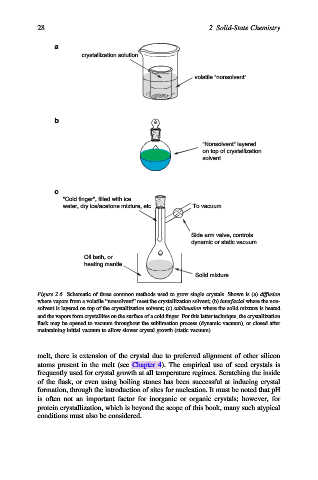
a
crystallization solution
volatile "nonsolvent"
b
"Nonsolvent" layered
on top of crystallization
solvent
c
"Cold finger", filled with ice
water, dry ice/acetone mixture, etc. To vacuum
Side arm valve, controls
dynamic or static vacuum
Oil bath, or
heating mantle
Solid mixture
Figure 2.6. Schematic of three common methods used to grow single crystals. Shown is (a) diffusion
where vapors from a volatile “nonsolvent” meet the crystallization solvent; (b) interfacial where the non-
solvent is layered on top of the crystallization solvent; (c) sublimation where the solid mixture is heated
and the vapors form crystallites on the surface of a cold finger. For this latter technique, the crystallization
flask may be opened to vacuum throughout the sublimation process (dynamic vacuum), or closed after
maintaining initial vacuum to allow slower crystal growth (static vacuum).
melt, there is extension of the crystal due to preferred alignment of other silicon
atoms present in the melt (see Chapter 4). The empirical use of seed crystals is
frequently used for crystal growth at all temperature regimes. Scratching the inside
of the flask, or even using boiling stones has been successful at inducing crystal
formation, through the introduction of sites for nucleation. It must be noted that pH
is often not an important factor for inorganic or organic crystals; however, for
protein crystallization, which is beyond the scope of this book, many such atypical
conditions must also be considered.