Page 45 - Materials Chemistry, Second Edition
P. 45
32 2 Solid-State Chemistry
integers such as (011) indicates that the crystallographic axis is intercepted in the
negative region (interception of the c axis at 1, in this case). Symmetry-equivalent
planes are indicated by {hkl}; for example, {100} for a cubic unit cell would have
six equivalent planes: (100), (100), (010), (010), (001), and (001).
Crystallographic directions correspond to vectors between two lattice points.
To properly index directions, the vector should first be (re)positioned so its starting
point is at (0,0,0) of the unit cell. The vector is then projected onto each of the three
crystallographic axes, multiplying/dividing by a common factor to remove frac-
tional terms. The three terms, expressed as integers, are enclosed within square
brackets of the form [abc] (Figure 2.10e). For instance, the [211] direction would
correspond to the vector a þ (1/2)b þ (1/2)c. Families of equivalent directions are
indicated by <abc>. For example, [100], [100], [010], [010], [001], and [001]
directions in a cubic crystal are designated as <100>.
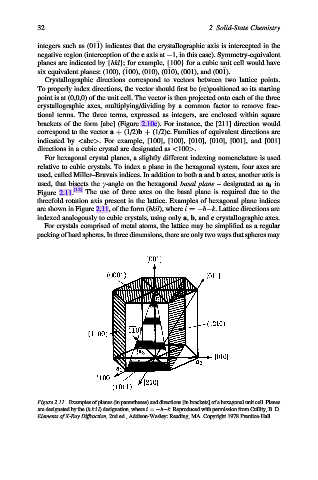
For hexagonal crystal planes, a slightly different indexing nomenclature is used
relative to cubic crystals. To index a plane in the hexagonal system, four axes are
used, called Miller–Bravais indices. In addition to both a and b axes, another axis is
used, that bisects the g-angle on the hexagonal basal plane – designated as a 3 in
Figure 2.11. [12] The use of three axes on the basal plane is required due to the
threefold rotation axis present in the lattice. Examples of hexagonal plane indices
are shown in Figure 2.11, of the form (hkil), where i ¼ h k. Lattice directions are
indexed analogously to cubic crystals, using only a, b, and c crystallographic axes.
For crystals comprised of metal atoms, the lattice may be simplified as a regular
packing of hard spheres. In three dimensions, there are only two ways that spheres may
Figure 2.11. Examples of planes (in parentheses) and directions [in brackets] of a hexagonal unit cell. Planes
are designated by the (hk i l) designation, where i ¼ h k. Reproduced with permission from Cullity, B. D.
Elements of X-Ray Diffraction, 2nd ed., Addison-Wesley: Reading, MA. Copyright 1978 Prentice-Hall.