Page 48 - Materials Chemistry, Second Edition
P. 48
35
2.3. The Crystalline State
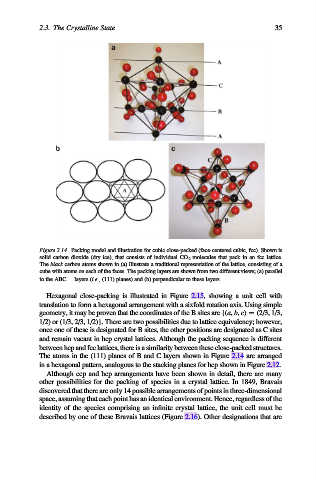
Figure 2.14. Packing model and illustration for cubic close-packed (face-centered cubic, fcc). Shown is
solid carbon dioxide (dry ice), that consists of individual CO 2 molecules that pack in an fcc lattice.
The black carbon atoms shown in (a) illustrate a traditional representation of the lattice, consisting of a
cube with atoms on each of the faces. The packing layers are shown from two different views; (a) parallel
to the ABC.. . layers (i.e., (111) planes) and (b) perpendicular to these layers.
Hexagonal close-packing is illustrated in Figure 2.15, showing a unit cell with
translation to form a hexagonal arrangement with a sixfold rotation axis. Using simple
geometry, it may be proven that the coordinates of the B sites are {(a, b, c) ¼ (2/3, 1/3,
1/2) or (1/3, 2/3, 1/2)}. There are two possibilities due to lattice equivalency; however,
once one of these is designated for B sites, the other positions are designated as C sites
and remain vacant in hcp crystal lattices. Although the packing sequence is different
between hcp and fcc lattices, there is a similarity between these close-packed structures.
The atoms in the (111) planes of B and C layers shown in Figure 2.14 are arranged
in a hexagonal pattern, analogous to the stacking planes for hcp shown in Figure 2.12.
Although ccp and hcp arrangements have been shown in detail, there are many
other possibilities for the packing of species in a crystal lattice. In 1849, Bravais
discovered that there are only 14 possible arrangements of points in three-dimensional
space, assuming that each point has an identical environment. Hence, regardless of the
identity of the species comprising an infinite crystal lattice, the unit cell must be
described by one of these Bravais lattices (Figure 2.16). Other designations that are