Page 71 - Mechanical Behavior of Materials
P. 71
70 Chapter 3 A Survey of Engineering Materials
25
160 158 114 Ni 20
Sn
Be
σ o , Yield Strength, MPa 120 117 143 125 Zn 15 σ o , ksi
Al
Si
80
10
133
40
5
Cu, 128 pm
0
0 10 20 30
Percent Solute
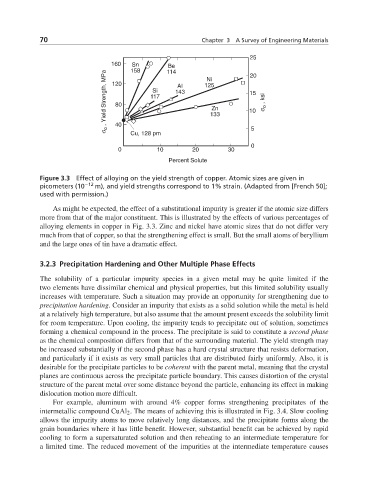
Figure 3.3 Effect of alloying on the yield strength of copper. Atomic sizes are given in
picometers (10 −12 m), and yield strengths correspond to 1% strain. (Adapted from [French 50];
used with permission.)
As might be expected, the effect of a substitutional impurity is greater if the atomic size differs
more from that of the major constituent. This is illustrated by the effects of various percentages of
alloying elements in copper in Fig. 3.3. Zinc and nickel have atomic sizes that do not differ very
much from that of copper, so that the strengthening effect is small. But the small atoms of beryllium
and the large ones of tin have a dramatic effect.
3.2.3 Precipitation Hardening and Other Multiple Phase Effects
The solubility of a particular impurity species in a given metal may be quite limited if the
two elements have dissimilar chemical and physical properties, but this limited solubility usually
increases with temperature. Such a situation may provide an opportunity for strengthening due to
precipitation hardening. Consider an impurity that exists as a solid solution while the metal is held
at a relatively high temperature, but also assume that the amount present exceeds the solubility limit
for room temperature. Upon cooling, the impurity tends to precipitate out of solution, sometimes
forming a chemical compound in the process. The precipitate is said to constitute a second phase
as the chemical composition differs from that of the surrounding material. The yield strength may
be increased substantially if the second phase has a hard crystal structure that resists deformation,
and particularly if it exists as very small particles that are distributed fairly uniformly. Also, it is
desirable for the precipitate particles to be coherent with the parent metal, meaning that the crystal
planes are continuous across the precipitate particle boundary. This causes distortion of the crystal
structure of the parent metal over some distance beyond the particle, enhancing its effect in making
dislocation motion more difficult.
For example, aluminum with around 4% copper forms strengthening precipitates of the
intermetallic compound CuAl 2 . The means of achieving this is illustrated in Fig. 3.4. Slow cooling
allows the impurity atoms to move relatively long distances, and the precipitate forms along the
grain boundaries where it has little benefit. However, substantial benefit can be achieved by rapid
cooling to form a supersaturated solution and then reheating to an intermediate temperature for
a limited time. The reduced movement of the impurities at the intermediate temperature causes