Page 55 - Mechanical Engineers' Handbook (Volume 4)
P. 55
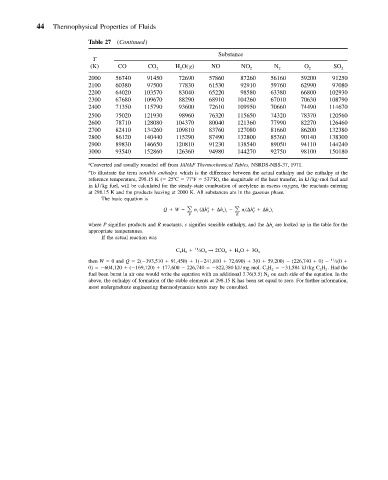
44 Thermophysical Properties of Fluids
Table 27 (Continued)
Substance
T
(K) CO CO 2 H 2 O(g) NO NO 2 N 2 O 2 SO 2
2000 56740 91450 72690 57860 87260 56160 59200 91250
2100 60380 97500 77830 61530 92910 59760 62990 97080
2200 64020 103570 83040 65220 98580 63380 66800 102930
2300 67680 109670 88290 68910 104260 67010 70630 108790
2400 71350 115790 93600 72610 109950 70660 74490 114670
2500 75020 121930 98960 76320 115650 74320 78370 120560
2600 78710 128080 104370 80040 121360 77990 82270 126460
2700 82410 134260 109810 83760 127080 81660 86200 132380
2800 86120 140440 115290 87490 132800 85360 90140 138300
2900 89830 146650 120810 91230 138540 89050 94110 144240
3000 93540 152860 126360 94980 144270 92750 98100 150180
a
Converted and usually rounded off from JANAF Thermochemical Tables, NSRDS-NBS-37, 1971.
b To illustrate the term sensible enthalpy, which is the difference between the actual enthalpy and the enthalpy at the
reference temperature, 298.15 K ( 25 C 77 F 537 R), the magnitude of the heat transfer, in kJ/kg mol fuel and
in kJ/kg fuel, will be calculated for the steady-state combustion of acetylene in excess oxygen, the reactants entering
at 298.15 K and the products leaving at 2000 K. All substances are in the gaseous phase.
The basic equation is
Q W n ( h h )
si
i ƒ si n ( h h )
ƒ
i
P R
where P signifies products and R reactants, s signifies sensible enthalpy, and the h s are looked up in the table for the
appropriate temperatures.
If the actual reaction was
CH 11 ⁄2O → 2CO HO 3O 2
2
2
2
2
2
then W 0 and Q 2( 393,510 91,450) 1( 241,810 72,690) 3(0 59,200) (226,740 0) 11 ⁄2(0
0) 604,120 ( 169,120) 177,600 226,740 822,380 kJ/mg mol. C 2 H 2 31,584 kJ/kg C 2 H 2 . Had the
fuel been burnt in air one would write the equation with an additional 3.76(5.5) N 2 on each side of the equation. In the
above, the enthalpy of formation of the stable elements at 298.15 K has been set equal to zero. For further information,
most undergraduate engineering thermodynamics texts may be consulted.