Page 52 - Mechanical Engineers' Handbook (Volume 4)
P. 52
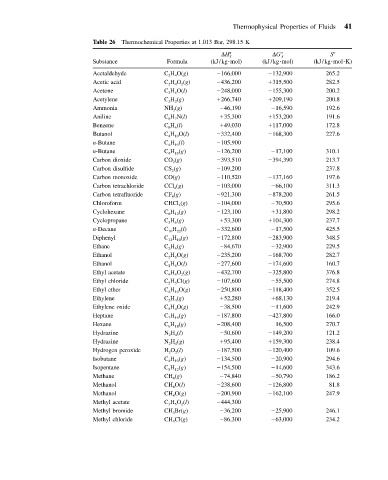
Thermophysical Properties of Fluids 41
Table 26 Thermochemical Properties at 1.013 Bar, 298.15 K
S
H ƒ G ƒ
Substance Formula (kJ/kg mol) (kJ/kg mol) (kJ/kg mol K)
Acetaldehyde C 2 H 4 O(g) 166,000 132,900 265.2
Acetic acid C 2 H 4 O 2 (g) 436,200 315,500 282.5
Acetone C 3 H 6 O(l) 248,000 155,300 200.2
Acetylene C 2 H 2 (g) 266,740 209,190 200.8
Ammonia NH 3 (g) 46,190 16,590 192.6
Aniline C 6 H 7 N(l) 35,300 153,200 191.6
Benzene C 6 H 6 (l) 49,030 117,000 172.8
Butanol C 4 H 10 O(l) 332,400 168,300 227.6
n-Butane C 4 H 10 (l) 105,900
n-Butane C 4 H 10 (g) 126,200 17,100 310.1
Carbon dioxide CO 2 (g) 393,510 394,390 213.7
Carbon disulfide CS 2 (g) 109,200 237.8
Carbon monoxide CO(g) 110,520 137,160 197.6
Carbon tetrachloride CCl 4 (g) 103,000 66,100 311.3
Carbon tetrafluoride CF 4 (g) 921,300 878,200 261.5
Chloroform CHCl 3 (g) 104,000 70,500 295.6
Cyclohexane C 6 H 12 (g) 123,100 31,800 298.2
Cyclopropane C 3 H 6 (g) 53,300 104,300 237.7
n-Decane C 10 H 22 (l) 332,600 17,500 425.5
Diphenyl C 12 H 10 (g) 172,800 283,900 348.5
Ethane C 2 H 6 (g) 84,670 32,900 229.5
Ethanol C 2 H 6 O(g) 235,200 168,700 282.7
Ethanol C 2 H 6 O(l) 277,600 174,600 160.7
Ethyl acetate C 4 H 8 O 2 (g) 432,700 325,800 376.8
Ethyl chloride C 2 H 5 Cl(g) 107,600 55,500 274.8
Ethyl ether C 4 H 10 O(g) 250,800 118,400 352.5
Ethylene C 2 H 4 (g) 52,280 68,130 219.4
Ethylene oxide C 2 H 4 O(g) 38,500 11,600 242.9
Heptane C 7 H 16 (g) 187,800 427,800 166.0
Hexane C 6 H 18 (g) 208,400 16,500 270.7
Hydrazine N 2 H 4 (l) 50,600 149,200 121.2
Hydrazine N 2 H 4 (g) 95,400 159,300 238.4
Hydrogen peroxide H 2 O 2 (l) 187,500 120,400 109.6
Isobutane C 4 H 10 (g) 134,500 20,900 294.6
Isopentane C 5 H 12 (g) 154,500 14,600 343.6
Methane CH 4 (g) 74,840 50,790 186.2
Methanol CH 4 O(l) 238,600 126,800 81.8
Methanol CH 4 O(g) 200,900 162,100 247.9
Methyl acetate C 3 H 6 O 2 (l) 444,300
Methyl bromide CH 3 Br(g) 36,200 25,900 246.1
Methyl chloride CH 3 Cl(g) 86,300 63,000 234.2