Page 53 - Mechanical Engineers' Handbook (Volume 4)
P. 53
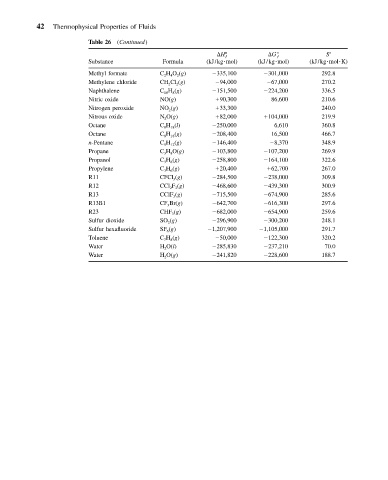
42 Thermophysical Properties of Fluids
Table 26 (Continued)
S
H ƒ G ƒ
Substance Formula (kJ/kg mol) (kJ/kg mol) (kJ/kg mol K)
Methyl formate C 2 H 4 O 2 (g) 335,100 301,000 292.8
Methylene chloride CH 2 Cl 2 (g) 94,000 67,000 270.2
Naphthalene C 10 H 8 (g) 151,500 224,200 336.5
Nitric oxide NO(g) 90,300 86,600 210.6
Nitrogen peroxide NO 2 (g) 33,300 240.0
Nitrous oxide N 2 O(g) 82,000 104,000 219.9
Octane C 8 H 18 (l) 250,000 6,610 360.8
Octane C 8 H 18 (g) 208,400 16,500 466.7
n-Pentane C 5 H 12 (g) 146,400 8,370 348.9
Propane C 3 H 8 O(g) 103,800 107,200 269.9
Propanol C 3 H 8 (g) 258,800 164,100 322.6
Propylene C 3 H 6 (g) 20,400 62,700 267.0
R11 CFCl 3 (g) 284,500 238,000 309.8
R12 CCl 2 F 2 (g) 468,600 439,300 300.9
R13 CClF 3 (g) 715,500 674,900 285.6
R13B1 CF 3 Br(g) 642,700 616,300 297.6
R23 CHF 3 (g) 682,000 654,900 259.6
Sulfur dioxide SO 2 (g) 296,900 300,200 248.1
Sulfur hexafluoride SF 6 (g) 1,207,900 1,105,000 291.7
Toluene C 7 H 8 (g) 50,000 122,300 320.2
Water H 2 O(l) 285,830 237,210 70.0
Water H 2 O(g) 241,820 228,600 188.7