Page 121 - Mechanism and Theory in Organic Chemistry
P. 121
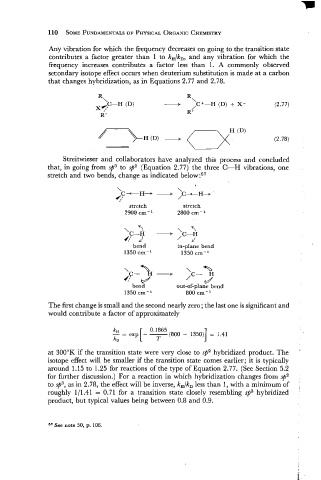
Any vibration for which the frequency decreases on going to the transition state
contributes a factor greater than 1 to kH/kD, and any vibration for which the
frequency increases contributes a factor less than 1. A commonly observed
secondary isotope effect occurs when deuterium substitution is made at a carbon
that changes hybridization, as in Equations 2.77 and 2.78.
R
\c+-H (D) + X- (2.77)
/
R '
H " " (D) + (-xH (D' (2.78)
Streitwieser and collaborators have analyzed this process and concluded
that, in going from sp3 to sp2 (Equation 2.77) the three C-H vibrations, one
stretch and two bends, change as indicated below:57
stretch stretch
2900 cm-l 2800 cm- '
bend in-plane bend
1350 cm-l 1350 cm-l
\ a
,C- H
d
bend out-of-plane bend
The first change is small and the second nearly zero; the last one is significant and
would contribute a factor of approximately
.
at 300°K if the transition state were very close to sp2 hybridized product. The
isotope effect will be smaller if the transition state comes earlier; it is typically
around 1.15 to 1.25 for reactions of the type of Equation 2.77. (See Section 5.2
for further discussion.) For a reaction in which hybridization changes from sp2
to sp3, as in 2.78, the effect will be inverse, kH/kD less than 1, with a minimum of
roughly 111.41 = 0.71 for a transition state closely resembling sp3 hybridized
product, but typical values being between 0.8 and 0.9.
See note 50, p. 106.