Page 116 - Mechanism and Theory in Organic Chemistry
P. 116
Isotope Effects 105
I
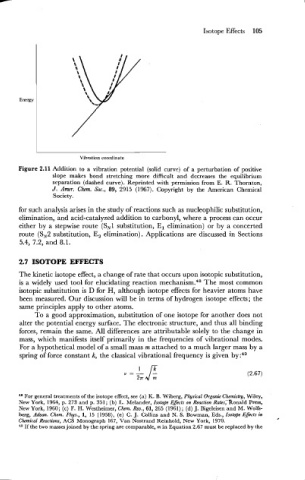
Vibration coordinate
Figure 2.11 Addition to a vibration potential (solid curve) of a perturbation of positive
slope makes bond stretching more difficult and decreases the equilibrium
separation (dashed curve). Reprinted with permission from E. R. Thornton,
J. Amer. Chnn. Soc., 89, 2915 (1967). Copyright by the American Chemical
Society.
for such analysis arises in the study of reactions such as nucleophilic substitution,
elimination, and acid-catalyzed addition to carbonyl, where a process can occur
either by a stepwise route (S,l substitution, E, elimination) or by a concerted
route (S,2 substitution, E, elimination). Applications are discussed in Sections
5.4, 7.2, and 8.1.
2.7 ISOTOPE EFFECTS
The kinetic isotope effect, a change of rate that occurs upon isotopic substitution,
is a widely used tool for elucidating reaction mechani~m.~~ The most common
isotopic substitution is D for H, although isotope effects for heavier atoms have
been measured. Our discussion will be in terms of hydrogen isotope effects; the
same principles apply to other atoms.
To a good approximation, substitution of one isotope for another does not
alter the potential energy surface. The electronic structure, and thus all binding
forces, remain the same. All differences are attributable solely to the change in
mass, which manifests itself primarily in the frequencies of vibrational modes.
For a hypothetical model of a small mass m attached to a much larger mass by a
spring of force constant k, the classical vibrational frequency is given by:49
48 For general treatments of the isotope effect, see (a) K. B. Wiberg, Physical Organit Chemistry, Wiley,
New York, 1964, p. 273 and p. 351; (b) L. Melander, Isotope Effects on Reaction Rates;Ronald Press,
New York, 1960; (c) F. H. Westheimer, Chem. Rev., 61, 265 (1961); (d) J. Bigeleisen and M. Wolfs-
berg, Advan. Chem. Phys., 1, 15 (1958), (e) C. J. Collins and N. S. Bowman, Eds., Isotope Effects in
Chemical Reactions, ACS Monograph 167, Van Nostrand Reinhold, New York, 1970.
48 If the two masses joined by the spring are comparable, m in Equation 2.67 must be replaced by the