Page 112 - Mechanism and Theory in Organic Chemistry
P. 112
Interpretation of Rate Constants 101
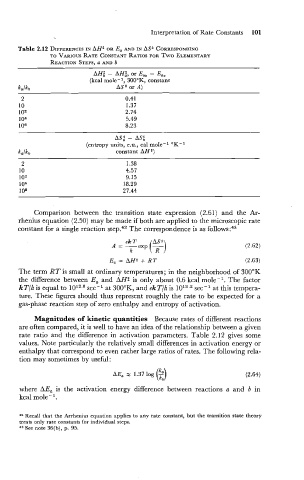
Table 2.12 DIFFERENCES AH* OR E, AND IN AS* CORRESPONDING
IN
TO VARIOUS RATE CONSTANT RATIOS FOR TWO ELEMENTARY
REACTION STEPS, a AND b
AH: - AH:, or Eab - Eaa
(kcal mole-l, 300°K, constant
ka/kb AS* or A)
2 0.41
10 1.37
1 Oa 2.74
1 04 5.49
10% 8.23
AS: - AS;
(entropy units, e.u., cal mole-l OK-l
constant AH *)
Comparison between the transition state expression (2.61) and the Ar-
rhenius equation (2.50) may be made if both are applied to the microscopic rate
constant for a single reaction step.42 The correspondence is as follows:43
E, = AH$ + RT (2.63)
The term RT is small at ordinary temperatures; in the neighborhood of 300°K
the difference between Ea and AHS is only about 0.6 kcal molep1. The factor
kT/h is equal to 1 012.8 sec-l at 300°K, and ek T/h is 1013.2 sec -I at this tempera-
ture. These figures should thus represent roughly the rate to be expected for a
gas-phase reaction step of zero enthalpy and entropy of activation.
Magnitudes of kinetic quantities Because rates of different reactions
are often compared, it is well to have an idea of the relationship between a given
rate ratio and the difference in activation parameters. Table 2.12 gives some
values. Note particularly the relatively small differences in activation energy or
enthalpy that correspond to even rather large ratios of rates. The following rela-
tion may sometimes by useful:
where AEa is the activation energy difference between reactions a and b in
kcal mole- l.
42 Recall that the Arrhenius equation applies to any rate constant, but the transition state theory
treats only rate constants for individual steps.
43 See note 36(b), p. 95.