Page 113 - Mechanism and Theory in Organic Chemistry
P. 113
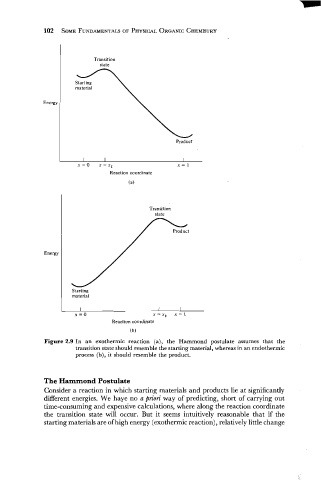
Transition
state
material
Product
Reaction coordinate
(a)
Transition
state
Energy
Starting
material
I I I
x=o x=x* I= I
Reaction coordinate
('J)
Figure 2.9 In an exothermic reaction (a), the Hammond postulate assumes that the
transition state should resemble the starting material, whereas in an endothermic
process (b), it should resemble the product.
The Hammond Postulate
Consider a reaction in which starting materials and products lie at significantly
different energies. We haye no a priori way of predicting, short of carrying out
time-consuming and expensive calculations, where along the reaction coordinate
the transition state will occur. But it seems intuitively reasonable that if the
starting materials are of high energy (exothermic reaction), relatively little change