Page 173 - Mechanism and Theory in Organic Chemistry
P. 173
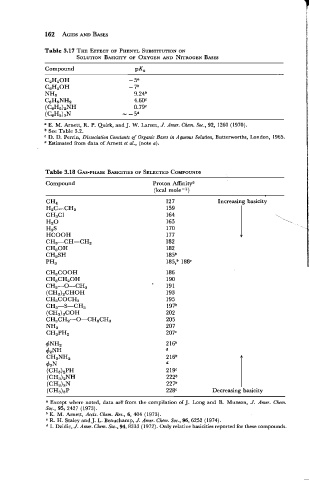
Table 3.17 THE EFFECT OF PHENYL SUBSTITUTION
ON
SOLUTION BASICITY OF OXYGEN AND NITROGEN BASES
Compound PKU
a E. M. Arnett, R. P. Quirk, and J. W. Larsen, J. Amer. Chem. SOC., 92, 1260 (1970).
See Table 3.2.
D. D. Perrin, Dissociation Constants of Organic Bases in Aqueous Solution, Butterworths, London, 1965.
Estimated from data of Arnett et al., (note a).
Table 3.18 GAS-PIIASE BASICITIES SELECTED COMPOUNDS
OF
Compound Proton Affinitya
(kcal mole- l)
CH4 127 Increasing basicity
H2C=CH2 159
CH3Cl 164 \
Hz0 165
H2S 170
HCOOH 177
CH3-CH=CH2 182
CH30H 182
CH3SH 185b
pH3 185,b 188'
CH3COOH
CH3CH20H
CH3-0-CH3
(CH3),CHOH
CH3COCH3
CH3-S-CH3
(CH3)3COH
CH3CH2-0-CH2CH3
NH3
CH3PH2
4NH2 216b
d
42NH
CH3NH2 216b
43N d
(CH3)2PH 219'
(CH3)zNH 2228
(CH3)3N 227b
(CH3)3P 228' Decreasing basicity
a Except where noted, data arE from the compilation of J. Long and B. Munson, J. Amer. Chem.
Sod., 95, 2427 (1973).
E. M. Arnett, Accts. Chem. Res., 6, 404 (1973).
R. H. Staley and J. L. Beauchamp, J. Amer. Chem. Sod., 96,6252 (1974).
I. Dzidic, J. Amer. Chem. Soc., 94,8333 (1972). Only relative basicities reported for these compounds.