Page 171 - Mechanism and Theory in Organic Chemistry
P. 171
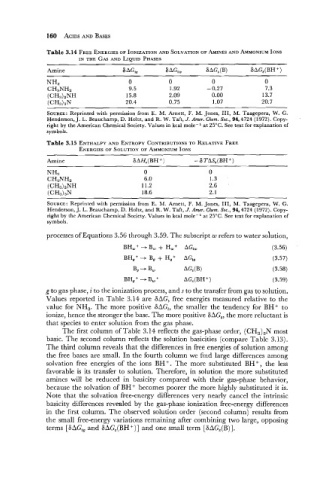
Table 3.14 FREE ENERGIES IONIZATION AND SOLVATION AMINES AND AMMONIUM IONS
OF
OF
IN THE GAS AND LIQUID PHASES
Amine 6 AGi, 6AGiw ~AGs(B) 6AGS(BHC)
NH3 0 0 0 0
CH3NHz 9.5 1.92 - 0.27 7.3
(CH3)zNH 15.8 2.09 0.00 13.7
(CH3)3N 20.4 0.75 1.07 20.7
SOURCE: Reprinted with permission from E. M. Arnett, F. M. Jones, 111, M. Taagepera, W. G.
Henderson, J. L. Beauchamp, D. Holtz, and R. W. Taft, J. Amer. Chem. Soc., 94,4724 (1972). Copy-
right by the American Chemical Society. Values in kcal mole- l at 25°C. See text for explanation of
symbols.
Table 3.15 ENTHALPY AND ENTROPY CONTRIBUTIONS RELATIVE FREE
TO
ENERGIES SOLUTION OF AMMONIUM IONS
OF
- -
Amine
NH3 0 0
CH3NH2 6.0 1.3
(CH3)2NH 11.2 2.6
(CH3)3N 18.6 2.1
SOURCE: Reprinted with permission from E. RI. Arnett, F. M. Jones, 111, M. Taagepera, W. G.
Henderson, J. L. Beauchamp, D, Holtz, and R. W. Taft, J. Amer. Chem. Soc., 94,4724 (1972). Copy-
right by the American Chemical Society. Values in kcal mole-' at 25°C. See text for explanation of
symbols.
processes of Equations 3.56 through 3.59. Thc subscript w refers to water solution,
AG,(BH + ) (3.59)
BH, + -+ Bw +
g to gas phase, i to the ionization process, and s to the transfer from gas to solution.
Values reported in Table 3.14 are SAG, free energies measured relative to the
value for NH,. The more positive SAGi, the smaller the tendency for BH+ to
ionize, hence the stronger the base. The more positive SAG,, the more reluctant is
that species to enter solution from thc gas phase.
The first column of Table 3.14 reflects the gas-phase order, (CH,),N most
basic. The second column reflects the solution basicities (compare Table 3.13).
The third column reveals that the differences in free energies of solution among
the free bases are small. In the fourth column we find large differences among
solvation free energies of the ions BH+. The more substituted BH+, the less
favorable is its transfer to solution. Therefore, in solution the more substituted
amincs will be reduced in basicity compared with their gas-phase behavior,
because the solvation of BH+ becomes poorer the more highly substituted it is.
Note that the solvation free-cncrgy differences very nearly cancel the intrinsic
basicity differences revealed by the gas-phase ionization free-energy differences
in the first column. The observed solution order (second column) results from
the small free-energy variations remaining after combining two large, opposing
terms [SAG,, and SAG,(BH+)] and one small term [GAG,(B)].