Page 167 - Mechanism and Theory in Organic Chemistry
P. 167
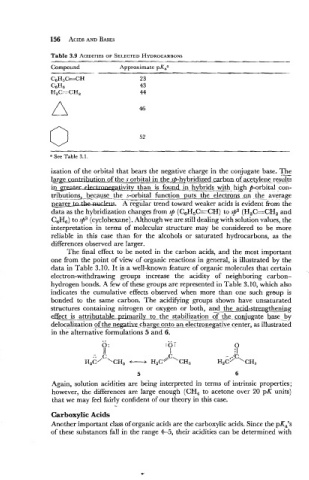
Table 3.9 ACIDITIES SELECTED HYDROCARBONS
OF
Compound Approximate pKaa
CBH5bCH 23
GH6 43
H,C--CH, 44
See Table 3.1
ization of the orbital that bears the negative charge in the conjugate base. The
1-tion of the s orbital in the sp-hybridized ~hanhan~fa~ee~1eene,~esu~s
in ativit than is found in hybrids .with- high p-orbital con-
n
t ? ~ o puts the electrons..-QV tk, average
pearer-. A regular trend toward weaker acids is evident from the
data as the hybridization changes from sp (C6H,C-CH) to sp2 (H2C=CH2 and
C6H6) to sp3 (cyclohexane). Although we are still dealing with solution values, the
interpretation in terms of molecular structure may be considered to be more
reliable in this case than for the alcohols or saturated hydrocarbons, as the
differences observed are larger.
The final effect to be noted in the carbon acids, and the most important
one from the point of view of organic reactions in general, is illustrated by the
data in Table 3.10. It is a well-known feature of organic molecules that certain
electron-withdrawing groups increase the acidity of neighboring carbon-
hydrogen bonds. A few of these groups are represented in Table 3.10, which also
indicates the cumulative effects observed when more than one such grsup is
bonded to the same carbon. The acidifying groups shown have unsaturated
structures containing nitrogen or oxygen or both, and the aci&strmgthenirrg
&ect is attributable primarily to the stabilization of the .- mugate base ... by
delocalizationof the negat~c~.ch~~n_to..annel~ectrone&atiiv.eecenter, illustrated
as
in the alternative formulations 5 and 6.
Again, solution acidities are being interpreted in terms of intrinsic properties;
however, the differences are large enough (CH, to acetone over 20 pK units)
that we may feel fairly confident of our theory in this case.
Carboxylic Acids
Another important class of organic acids are the carboxylic acids. Since the pK,'s
of these substances fall in the range 4-5, their acidities can be determined with