Page 169 - Mechanism and Theory in Organic Chemistry
P. 169
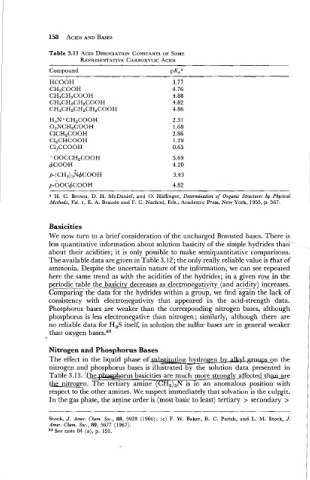
Table 3.11 ACID DISSOCIATION CONSTANTS SOME
OF
CARBOXYLIC ACIDS
REPRESENTATIVE
Compound pKaa
HCOOH
CH3COOH
CH3CH2COOH
CH3CH2CH2COOH
CH3CH2CHzCH2COOH
H. C. Brown, D. H. McDaniel, and 0. Haflinger, Determination of Organic Structures by Physical
Methods, Vol. I, E. A. Braude and F. C. Nachod, Eds., Academic Press, New York, 1955, p. 567.
Basicities
We now turn to a brief consideration of the uncharged Brcansted bases. There is
less quantitative information about solution basicity of the simple hydrides thah'
about their acidities; it is only possible to make semiquantitative comparisons.
The available data are given in Table 3.12 ; the only really reliable value is that of
ammonia. Despite the uncertain nature of the information, we can see repeated
here the same trend as with the acidities of the hydrides; in a given row in the
_
periodic table the --- basicity decreases as elec~ro_negativity_ (and acidity). - . -- inc-reases.
_ ._
c;mparing the data for the hydrides within a group, we find again the lack of
consistency with electronegativity that appeared in the acid-strength data.
Phosphorus bases are weaker than the corresponding nitrogen bases, although
phosphorus is less electronegative than nitrogen; similarly, although there are
no reliable data for H,S itself, in solution the sulfur bases are in general weaker
than oxygen bases.93
Nitrogen and Phosphorus Bases
The effect in the liquid phase ofwtuting hydrogen by -on the
nitrogen and phosphorus bases is illustrated Fy the solution data presented in
ongly -affe_~te&h=e
nomalous position with
respect to the other amines. We suspect immediately that solvation is the culpjit.
In the gas phase, the amine order is (most basic to least) tertiary > secondary >
Stock, J. Amer. Chem. Soc., 88, 5928 (1966); (c) F. W. Baker, R. C. Parish, and L. M. Stock, J.
Amer. Chem. Soc., 89, 5677 (1967).
83 See note 84 (a), p. 151.