Page 162 - Mechanism and Theory in Organic Chemistry
P. 162
Substituent Effects on Strengths of Brcansted Acids and Bases 151
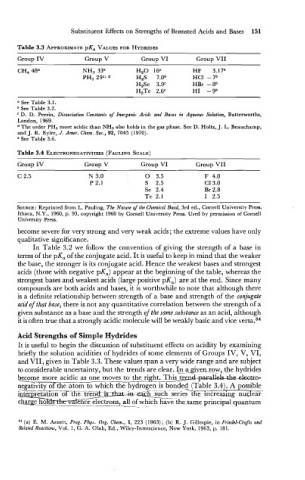
Table 3.3 APPROXIMATE pKa VALUES FOR HYDRIDES
Group IV Group V Group VI Group VII
CH, 48" NH, 33b H20 16e HE 3.17b
PH, 2gC. H2S 7.0b HCl -7b
H2Se 3.gC HBr -8b
H2Te 2.6C HI -9b
'I See Table 3.1.
See Table 3.2.
D. D. Perrin, Dissociation Constants of Inorganic Acids and Bases in Aqueous Solution, Butterworths,
London, 1969.
The order pH3 more acidic than NH3 also holds in the gas phase. See D. Holtz, J. L. Beauchamp,
and J. R. Eyler, J. Amer. Chem. Soc., 92, 7045 (1970).
" See Table 3.6.
Table 3.4 ELECTRONEGATIVITIES (PAULING SCALE)
Group IV Group V Group VI Group VII
SOURCE: Reprinted from L. Pauling, The Nalure of the Chemical Bond, 3rd ed., Cornell University Press,
Ithaca, N.Y., 1960, p. 93, copyright 1960 by Cornell University Press. Used by permission of Cornell
University Press.
become severe for very strong and very weak acids; the extreme values have only
qualitative significance.
In Table 3.2 we follow the convention of giving the strength of a base in
terms of the pKa of the conjugate acid. It is useful to keep in mind that the weaker
the base, the stronger is its conjugate acid. Hence the weakest bases and strongest
acids (those with negative pKa) appear at the beginning of the table, whereas the
strongest bases and weakest acids (large positive pKa) are at the end. Since many
compounds are both acids and bases, it is worthwhile to note that although there
is a definite relationship between strength of a base and strength of the conjugate
acid of that base, there is not any quantitative correlation between the strength of a
given substance as a base and the strength of the same substance as an acid, although
it is often true that a strongly acidic molecule will be weakly basic and vice versa.84
Acid Strengths of Simple Hydrides
It is useful to begin the discussion of substituent effects on acidity by examining
briefly the solution acidities of hydrides of some elements of Groups IV, V, VI,
and VII, given in Table 3.3. These values span a very wide range and are subject
to considerable uncertainty, but the trends are clear. I_n agi-ven row, the hydrides
become more acidic as one moves to the right. This treIxbarduea-
-
-
- - - . - -
-
negativity of the atom-hydrogen is bonded (Table 3.4). A possible
chse -= all .. -..-_ ~ . -
interprXiGii-6f -fie etw:inin~E -iikh'--i&rie<the increasing nuclear-
electrons ...... of which have the same principal quantum
ho
ence
-
-
-.
84 (a) E. M. Arnett, Pros. Phys. Org. Chem., 1, 223 (1963); (b) R. J. Gillespie, in Friedel-Crafls and
Related Reactions, Vol. 1, G. A. Olah, Ed., Wiley-Interscience, New York, 1963, p. 181.