Page 158 - Mechanism and Theory in Organic Chemistry
P. 158
Strengths of Weak Brernsted Acids 147
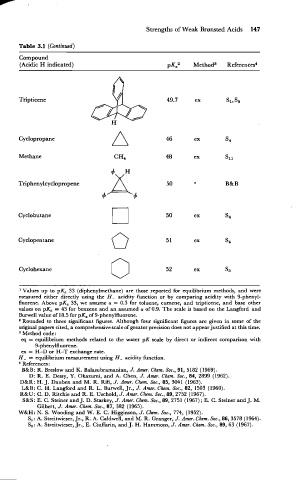
Table 3.1 (Continued)
Compound
(Acidic H indicated)
Cyclopropane
Methane
Cyclopentane 0 5 1 ex S4
Cyclohexane
Values up to pK, 33 (diphenylmethane) are those reported for equilibrium methods, and were
measured either directly using the H- acidity function or by comparing acidity with 9-phenyl-
fluorene. Above pK, 33, we assume a = 0.3 for toluene, cumene, and tripticene, and base other
values on pK, = 43 for benzene and an assumed a of 0.9. The scale is based on the Langford and
Burwell value of 18.5 for pKa of 9-phenylfluorene.
a Rounded to three significant figures. Although four significant figures are given in some of the
original papers cited, a comprehensive scale of greater precision does not appear justified at this time.
Method code:
eq = equilibrium methods related to the water pK scale by direct or indirect comparison with
9-phenylfluorene.
ex = H-D or H-T exchange rate.
H- = equilibrium measurement using H- acidity function.
References :
B&B: R. Breslow and K. Balasubramanian, J. Amer. Chem. Soc., 91, 5182 (1969).
D: R. E. Dessy, Y. Okazumi, and A. Chen, J. Am. Chem. Soc., 84, 2899 (1962).
D&R: H. J. Dauben and M. R. Rifi, J. Amer. Chem. Soc., 85, 3041 (1963).
L&B: C. H. Langford and R. L. Burwell, Jr., J. Amer. Chem. Soc., 82, 1503 (1960).
R&U: C. D. Ritchie and R. E. Uschold, J. Amer. Chem. Soc., 89, 2752 (1967).
S&S: E. C. Steiner and J. D. Starkey, J. Amer. Chem. Soc., 89,2751 (1967) ; E. C. Steiner and J. M.
Gilbert, J. Amer. Chem. Soc., 87, 382 (1965).
W&H: N. S. Wooding and W. E. C. Higginson, J. Chem. Soc., 774, (1952).
Sl: A. Streitwieser, Jr., R. A. Caldwell, and M. R. Granger, J. Amer. Chem. Soc., 86, 3578 (1964).
S2: A. Streitwieser, Jr., E. Ciuffarin, and J. H. Hammons, J. Amer. Chem. Soc., 89, 63 (1967).