Page 182 - Mechanism and Theory in Organic Chemistry
P. 182
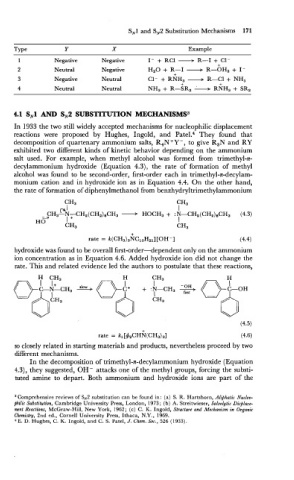
SN1 and SN2 Substitution Mechanisms 171
Type Y X Example
1 Negative Negative I- + RC1 ----t R-I + C1-
L
+
2 Neutral Negative H~O R-I + R- OH^ + I -
+
3 Negative Neutral C1- + RNH3 + R-Cl + NH3
+ +
4 Neutral Neutral NH3 + R-SR2 L RNH, + SR2
4.1 SN1 AND SN2 SUBSTITUTION MECHANISMS3
In 1933 the two still widely accepted mechanisms for nucleophilic displacement
reactions were proposed by Hughes, Ingold, and Patel.' They found that
decomposition of quartenary ammonium salts, R,N+Y -, to give R3N and RY
exhibited two different kinds of kinetic behavior depending on the ammonium
salt used. For example, when methyl alcohol was formed from trimethyl-n-
decylammonium hydroxide (Equation 4.3), the rate of formation of methyl
alcohol was found to be second-order, first-order each in trimethyl-n-decylam-
monium cation and in hydroxide ion as in Equation 4.4. On the other hand,
the rate of formation of diphenylmethanol from benzhydryltrimethylammonium
+
rate = k(CH3)3NCloH21] [OH-]
hydroxide was found to be overall first-order-dependent only on the ammonium
ion concentration as in Equation 4.6. Added hydroxide ion did not change the
rate. This and related evidence led the authors to postulate that these reactions,
+
rate = kl [+2CHN(CH3)3] (4.6)
so closely related in starting materials and products, nevertheless proceed by two
different mechanisms.
In the decomposition of trimethyl-n-decylammonium hydroxide (Equation
4.3), they suggested, OH- attacks one of the methyl groups, forcing the substi-
tuted amine to depart. Both ammonium and hydroxide ions are part of the
Comprehensive reviews of SN2 substitution can be found in: (a) S. R. Hartshorn, Aliphatic Nucleo-
philic Substitution, Cambridge University Press, London, 1973; (b) A. Streitwieser, Soluolytic Displace-
ment Reactions, McGraw-Hill, New York, 1962; (c) C. K. Ingold, Structure and Mechanism in Organic
Chemistry, 2nd ed., Cornell University Press, Ithaca, N.Y., 1969.
* E. D. Hughes, C. K. Ingold, and C. S. Patel, J. Chem. Soc., 526 (1933).