Page 184 - Mechanism and Theory in Organic Chemistry
P. 184
SN1 and SN2 Substitution Mechanisms 173
Reaction coordinate
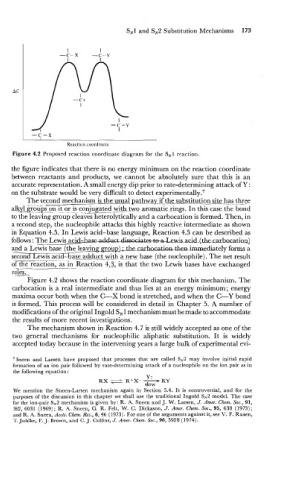
Figure 4.2 Proposed reaction coordinate diagram for the SN1 reaction.
the figure indicates that there is no energy minimum on the reaction coordinate
between reactants and products, we. cannot be absolutely sure that this is an
accurate representation. A small energy dip prior to rate-determining attack of Y:
on the substrate would be very difficult to detect e~perimentally.~
The second mechanism is the usualpath-w-ay-ifthe substit_ution site has-thtlee
is
alk~ro-ps-~_n it-or .. . conjugated with~wo aromatic rings. In this case the bond
.
.____IC_l---.---
to the leaving group cleaves heterolytically and a carbocation is formed. Then, in
a second step, the nucleophile attacks this highly reactive intermediate as shown
in Equation 4.5. In Lewis acid-base language, Reaction 4.5 can be described as
follows : The Lewis acid--st--(thembocatia~).
and a L~GS base (the leaving arou&,-&s&matkmthen-immediately forms a
~
f
~
i
d
~
~
~
~
s -f r----.----y. ~~~ - -. s ~ - danew base (the nucleophile). The net result h
~
-
o . t e reaction as in -- Reaction 4,3, is that the two Lewis bases have exchanged
-
.
rok
Figure 4.2 shows the reaction coordinate diagram for this mechanism. The
carbocation is a real intermediate and thus lies at an energy minimum; energy
maxima occur both when the C-X bond is stretched, and when the C-Y bond
is formed. This process will be considered in detail in Chapter 5. A number of
modifications of the original Ingold S, 1 mechanism must be made to accommodate
the results of more recent investigations.
The mechanism shown in Reaction 4.7 is still widely accepted as one of the
two general mechanisms for nucleophilic aliphatic substitution. It is widely
accepted today because in the intervening years a large bulk of experimental evi-
' Sneen and Larsen have proposed that processes that are called SN2 may involve initial rapid
formation of an ion pair followed by rate-determining attack of a nucleophile on the ion pair as in
the following equation:
-.
Y:
RX R+X-
ZZ- RY
We mention the Sneen-Larsen mechanism again in Section 5.4. It is controversial, and for the
purposes of the discussion in this chapter we shall use the traditional Ingold SN2 model. The case
for the ion-pair SN2 mechanism is given by: R. A. Sneen and J. W. Larsen, J. Amer. Chem. Soc., 91,
362, 6031 (1969); R. A. Sneen, G. R. Felt, W. C. Dickason, J. Amer. Chem. Soc., 95, 638 (1973);
and R. A. Sneen, Accts. Chem. Res., 6,46 (1973). For one of the arguments against it, see V. F. Raaen,
T. Juhlke, F. J. Brown, and C. J. Collins, J. Amer. Chem. Soc., 96, 5928 (1974).