Page 188 - Mechanism and Theory in Organic Chemistry
P. 188
The Solvent, Substrate, Nucleophile, and Leaving Group 177
4.3 THE SOLVENT, SUBSTRATE,
NUCLEOPHILE, AND LEAVING GROUP
The nature of the solvent and the structures of the substrate, nucleophile, and
leaving group all help determine whether a nucleophilic displacement proceeds
by a unimolecular or bimolecular pathway. They also all affect the rate of reac-
tion.
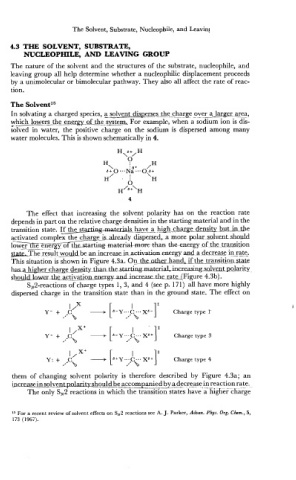
The Solvent15
In solvating a charged species, a solvent dispersss lhesharge over a largasea,
which lowers the energv of th_e-s_yst_em,. For-example, when asodium ion is dis-
--
c __; --
solved in water, the positive charge on the sodium is dispersed among many
water molecules. This is shown schematically in 4.
The effect that increasing the solvent polarity has on the reaction rate
depends in part on the relative charge densities in the starting material and in the
transition state. If the starting-als have a hi&-ch.a%e- de_nsk~ but-lhe
activated compl& the char-ge.is&cady dispersed, a mare polar dumLshaYld
1:~- ~n~fjgy-arti~ materia&- than the-ergy of thctxansition
-
and
$&&The result-woddbe anincreaseinactivati~~~mergy a decrease in rate.
This situation is shown in Figure 4.3a. Ogth-hand, if the traqsitio~~slate
-e-teri&hasing sd~en.Lp-carity
should hwx_&&c&ation r_atc(Figure 4.3b).
SN2-reactions of charge types 1, 3, and 4 (see p. 17 1) all have more highly
dispersed charge in the transition state than in the ground state. The effect on
them of changing solvent polarity is therefore described by Figure 4.3a; an
ibcrease in solvent palzixityshould be accomp_it.nied-by?-d~crease in reaction rate.
The only SN2 reactions in which the transition states have': higher charge
l5 For a recent review of solvent effects on S,2 reactions see A. J. Parker, Advan. Phys. Org. Chem., 5,
173 (1967).