Page 193 - Mechanism and Theory in Organic Chemistry
P. 193
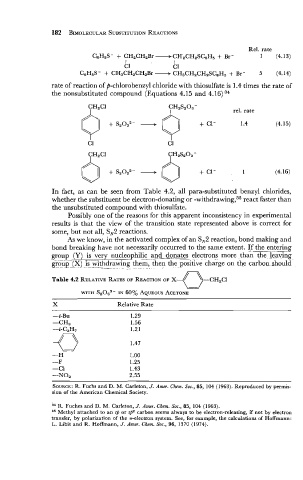
Rel. rate
CeH5S- + CH2CH2Br -CH2CH2SCeH5 + Br- 1 (4.13)
I I
C1 Cl
C&S- + CH3CH2CH2Br - CH3CH2CH2SCeH5 + Br- 5 (4.14)
rate of reaction of p-chlorobenzyl chloride with thiosulfate is 1.4 times the rate of
the nonsubstituted compound (Equations 4.15 and 4.16) 34
CHzCl CHzSz03 -
rel. rate
0 - 3 + 1.4 (4.15)
+
sz03z-
C1 C1
In fact, as can be seen from Table 4.2, all para-substituted benzyl chlorides,
whether the substituent be electron-donating or -withdrawing,35 react faster than
the unsubstituted compound with thiosulfate.
Possibly one of the reasons for this apparent inconsistency in experimental
results is that the view of the transition state represented above is correct for
some, but not all, SN2 reactions.
As we know, in the activated complex of an SN2 reaction, bond making and
bond breaking have not necessarily occurred to the same extent. If the entering
g-*P (Y) is nucleophilic and donates electrons more than the leaving
-----
grouplx) is withdrawing them, then the positive charge on the carbon should
- -
Table 4.2 RELATIVE RATES OF REACTION OF X-
w
WITH S2032- IN 60% AQUEOUS ACETONE
X Relative Rate
SOURCE: R. Fuchs and D. M. Carleton, J. Amer. Chern. SOC., 85, 104 (1963). Reproduced by permis-
sion of the American Chemical Society.
34 R. Fuches and D. M. Carleton, J. Amer. Chern. SOC., 85, 104 (1963).
35 Methyl attached to an sp or sp2 carbon seems always to be electron-releasing, if not by electron
transfer, by polarization of the r-electron system. See, for example, the calculations of Hoffmann:
L. Libit and R. Hoffmann, J. Amer. Chern. SOC., 96, 1370 (1974).