Page 195 - Mechanism and Theory in Organic Chemistry
P. 195
the rate (see Table 4.3). In these displacements thiophenoxide, which is an
excellent nucleophile (see p. 189), is always the attacking reagent and therefore
the results are not surprising within the context of the above argument.
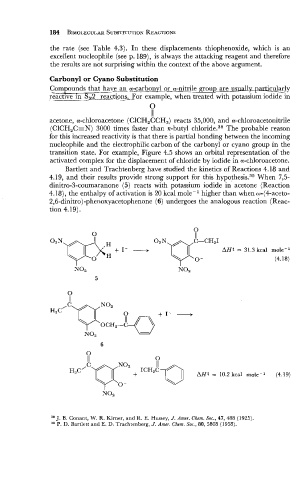
Carbonyl or Cyano Substitution
Compounds that have an_pCI_carbqn_rl_o~i~;legroup~icular!y
reactive in SN2 rxaction_s_For example, when treated with potassium iodide in
0
II
acetone, a-chloroacetone (C1CH2CCH3) reacts 35,000, and a-chloroacetonitrile
(C1CH2C-N) 3000 times faster than n-butyl chloride.38 The probable reason
for this increased reactivity is that there is partial bonding between the incoming
nucleophile and the electrophilic carbon of the carbonyl or cyano group in the
transition state. For example, Figure 4.5 shows an orbital representation of the
activated complex for the displacement of chloride by iodide in a-chloroacetone.
Bartlett and Trachtenberg have studied the kinetics of Reactions 4.18 and
4.19, and their results provide strong support for this hypothe~is.~~ When 7,5-
dinitro-3-coumaranone (5) reacts with potassium iodide in acetone (Reaction
4.18), the enthalpy of activation is 20 kcal molep1 higher than when w-(4-aceto-
2,6-dinitro)-phenoxyacetophenone (6) undergoes the analogous reaction (Reac-
tion 4.19).
0
I1
02N'7c-cH21
AHr = 31.3 kcal mole-l
0-
NO2 NO2 (4.18)
5
38 J. B. Conant, W. R. Kirner, and R. E. Hussey, J. Amer. Chem. Soc., 47, 488 (1925).
38 P. D. Bartlett and E. D. Trachtenberg, J. Amer. Chem. Soc., 80, 5808 (1958).