Page 199 - Mechanism and Theory in Organic Chemistry
P. 199
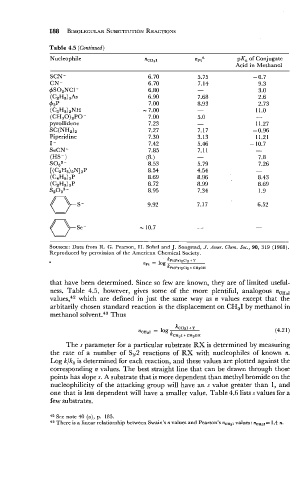
Table 4.5 (Continued)
Nucleophile ~CH,I npta pK, of Conjugate
Acid in Methanol
SCN -
CN-
4S02NCl -
(C2H5)3As
43p
(C2H5)2NH
(CH30),P0 -
pyrollidene
SC(NH212
Piperidine
I -
SeCN-
(HS-1
[(C2H5)2NI,P
(C4H9)3P
(C2H5)3P
S2032-
SOURCE: Data from R. G. Pearson, H. Sobel and J. Songstad, J. Amer. Chern. Soc., 90, 319 (1968).
Reproduced by permission of the American Chemical Society.
that have been determined. Since so few are known, they are of limited useful-
ness. Table 4.5, however, gives some of the more plentiful, analogous n,,,,
values,42 which are defined in just the same way as n values except that the
arbitarily chosen standard reaction is the displacement on CH31 by methanol in
methanol solvent.43 Thus
~CH~I+Y
(4.2 1)
ncH31 = log kc,,, + c,,,on
The s parameter for a particular substrate RX is determined by measuring
the rate of a number of S,2 reactions of RX with nucleophiles of known n.
Log klk, is determined for each reaction, and these values are plotted against the
corresponding n values. The best straight line that can be drawn through those
points has slope s. A substrate that is more dependent than methyl bromide on the
nucleophilicity of the attacking group will have an s value greater than 1, and
one that is less dependent will have a smaller value. Table 4.6 lists s values for a
few substrates.
42 See note 40 (a), p. 185.
43 There is a linear relationship between Swain's n values and Pearson's ncHal values: ncH3,= 1.4 n.