Page 200 - Mechanism and Theory in Organic Chemistry
P. 200
The Solvent, Substrate, Nucleophile, and Leaving Group 189
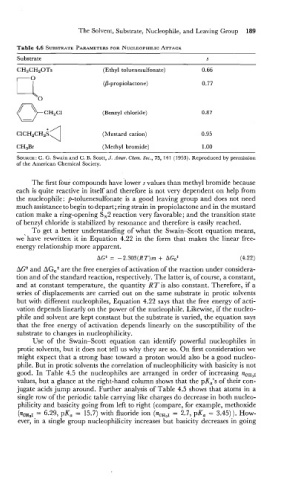
Table 4.6 SUBSTRATE PARAMETERS NUCLEOPHILIC ATTACK
FOR
Substrate s
CH3CH20Ts (Ethyl toluenesulfonate) 0.66
(Benzyl chloride)
'3
ClCH2CH2S (Mustard cation)
CH3Br (Methyl bromide)
SOURCE:
C. G. Swain and C. B. Scott, J. Amer. Chem. Soc., 75, 141 (1953). Reproduced by permission
of the American Chemical Society.
The first four compounds have'lower s values than methyl bromide because
each is quite reactive in itself and therefore is not very dependent on help from
the nucleophile: p-toluenesulfonate is a good leaving group and does not need
much assistance to begin to depart; ring strain in propiolactone and in the mustard
cation make a ring-opening S,2 reaction very favorable; and the transition state
of benzyl chloride is stabilized by resonance and therefore is easily reached.
To get a better understanding of what the Swain-Scott equation means,
we'have rewritten it in Equation 4.22 in the form that makes the linear free-
energy relationship more apparent.
AG* = -2.303(RT)sn + AGO* (4.22)
AG* and AGO* are the free energies of activation of the reaction under considera-
tion and of the standard reaction, respectively. The latter is, of course, a constant,
and at constant temperature, the quantity RT is also constant. Therefore, if a
series of displacements are carried out on the same substrate in protic solvents
but with different nucleophiles, Equation 4.22 says that the free energy of acti-
vation depends linearly on the power of the nucleophile. Likewise, if the nucleo-
phile and solvent are kept constant but the substrate is varied, the equation says
that the free energy of activation depends linearly on the susceptibility of the
substrate to changes in nucleophilicity.
Use of the Swain-Scott equation can identify powerful nucleophiles in
protic solvents, but it does not tell us why they are so. On first consideration we
might expect that a strong base toward a proton would also be a good nucleo-
phile. But in protic solvents the correlation of nucleophilicity with basicity is not
good. In Table 4.5 the nucleophiles are arranged in order of increasing n,,,,
values, but a glance at the right-hand column shows that the pK,'s of their con-
jugate acids jump around. Further analysis of Table 4.5 shows that atoms in a
single row of the periodic table carrying like charges do decrease in both nucleo-
philicity and basicity going from left to right (compare, for example, methoxide
(no,, = 6.29, pK, = 15.7) with fluoride ion (n,,,, = 2.7, pK, = 3.45)). How-
ever, in a single group nucleophilicity increases but basicity decreases in going