Page 205 - Mechanism and Theory in Organic Chemistry
P. 205
this charge type (see p. 178), changing from the polar solvent methanol to the
nonpolar solvent DMF greatly increases the rates of all the reactions. However,
note that in the series of displacements by azide ion, when the leaving group is
iodide, the rate is increased lo5 times by the solvent change; when it is bromide,
1.7 x lo4 times; and when chloride is departing, the factor is only 5 x lo3. A
similar series is observed when thiocyanate is the nucleophile. The explanation
for this is very similar to that given for the solvent dependence of nucleophilicity.
Apparently methanol is able to solvate the smaller activated complex
[Y .-.CH3--C1] - much better than it can [Y --CH3..-I] - . Therefore, although
changing solvents from DMF to methanol is unfavorable for all the reactions in
Table 4.8, it is not as unfavorable for methyl chloride, where the transition state
can be effectively solvated, as for methyl iodide, where it cannot.
4.4 BIMOLECULAR NUCLEOPHILIC SUBSTITUTION AT SULFUR54
There has been great interest in recent years in bimolecular nucleophilic displace-
ment reactions on organic compounds where the site of substitution is not carbon
but oxygen, sulfur, or silicon. Since there is not room to discuss each of these
reactions here, we shall briefly consider bimolecular nucleophilic displacements
on sulfur as an example and refer the reader to recent reviews of displacement at
oxygen55 and silicon.56
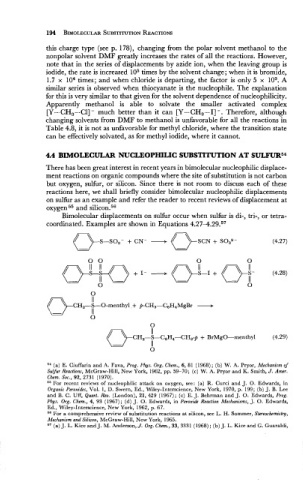
Bimolecular displacements on sulfur occur when sulfur is di-, tri-, or tetra-
coordinated. Examples are shown in Equations 4.27-4.29.57
54 (a) E. Ciuffarin and A. Fava, Prog. Phys. Org. Chem., 6, 81 (1968); (b) W. A. Pryor, Mechanism of
SuC/ur Reactions, McGraw-Hill, New York, 1962, pp. 59-70; (c) W. A. Pryor and K. Smith, J. Amer.
Chem. Soc., 92, 2731 (1970).
65 For recent reviews of nucleophilic attack on oxygen, see: (a) R. Curci and J. 0. Edwards, in
Organic Peroxides, Vol. 1, D. Swern, Ed., Wiley-Interscience, New York, 1970, p. 199; (b) J. B. Lee
and B. C. Uff, Quart. Rev. (London), 21, 429 (1967); (c) E. J. Behrman and J. 0. Edwards, Prog.
Phys. Org. Chem., 4, 93 (1967); (d) J. 0. Edwards, in Peroxide Reaction Mechanisms, J. 0. Edwards,
Ed., Wiley-Interscience, New York, 1962, ,p. 67.
56 For a comprehensive review of substitution reactions at silicon, see L. H. Sommer, Stereochemisty,
Mechanism and Silicon, McGraw-Hill, New York, 1965.
(a) J. L. Kice and J. M. Anderson, J. Org. Chem., 33, 333 1 (1968); (b) J. L. Kice and G. Guaraldi,