Page 209 - Mechanism and Theory in Organic Chemistry
P. 209
occur in resolvable enantioneric pairs, and those in which sulfur is part of a sub-
stituted ring system form isolable geometric (cis-trans) isomers. In most cases,
the product of a nucleophilic substitution on tricoordinated sulfur is found to
have the opposite configuration from the starting material.
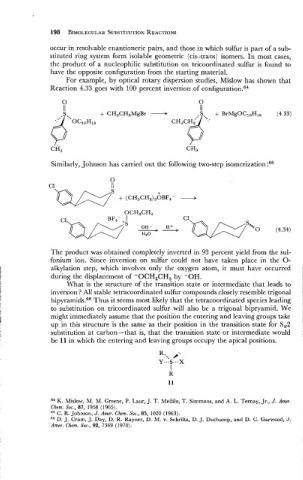
For example, by optical rotary dispersion studies, Mislow has shown that
Reaction 4.33 goes with 100 percent inversion of c~nfiguration.~~
Similarly, Johnson has carried out the following two-step isomeri~ation:~~
The product was obtained completely inverted in 93 percent yield from the sul-
fonium ion. Since inversion on sulfur could not have taken place in the 0-
alkylation step, which involves only the oxygen atom, it must have occurred
during the displacement of -OCH,CH, by -OH.
What is the structure of the transition state or intermediate that leads to
inversion ? All stable tetracoordinated sulfur compounds closely resemble trigonal
bipyramid~.~~ Thus it seems most likely that the tetracoordinated species leading
to substitution on tricoordinated sulfur will also be a trigonal bipryamid. We
might immediately assume that the position the entering and leaving groups take
up in this structure is the same as their position in the transition state for S,2
substitution at carbon-that is, that the transition state or intermediate would
be 11 in which the entering and leaving groups occupy the apical positions.
84 K. Mislow, M. M. Greene, P. Laur, J. T. Mellilo, T. Simmons, and A. L. Ternay, Jr., J. Amer.
Chem. SOC., 87, 1958 (1965).
O5 C. R. Johnson, J. Amer. Chem. SOC., 85, 1020 (1963).
D. J. Cram, J. Day, D. R. Rayner, D. M. v. Schriltz, D. J. Duchamp, and D. C. Garwood, J.
Amer. Chem. SOC., 92, 7369 (1970).