Page 210 - Mechanism and Theory in Organic Chemistry
P. 210
Bimolecular Nucleophilic Substitution at Sulfur 199
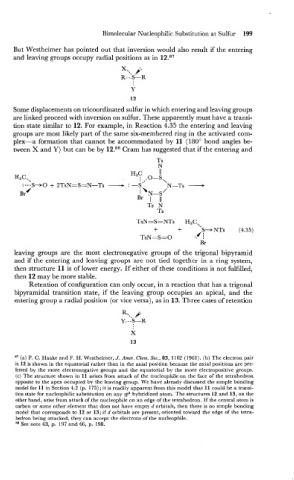
But Westheimer has pointed out that inversion would also result if the entering
and leaving groups occupy radial positions as in 12.67
X.,, j'
R-S-R
Some displacements on tricoordinated sulfur in which entering and leaving groups
are linked proceed with inversion on sulfur. These apparently must have a transi-
tion state similar to 12. For example, in Reaction 4.35 the entering and leaving
groups are most likely part of the same six-membered ring in the activated com-
plex-a formation that cannot be accommodated by 11 (180" bond angles be-
tween X and Y) but can be by 12.68 Cram has suggested that if the entering and
TsN=S=NTs H,C,
+ + S--t NTs (4.35)
./ :
TsN=S=O .,
Br
leaving groups are the most electronegative groups of the trigonal bipyramid
and if the entering and leaving groups are not tied together in a ring system,
then structure 11 is of lower energy. If either of these conditions is not fulfilled,
then 12 may be more stable.
Retention of configuration can only occur, in a reaction that has a trigonal
bipyramidal transition state, if the leaving group occupies an apical, and the
entering group a radial position (or vice versa), as in 13. Three cases of retention
(a) P. C. Haake and F. H. Westheimer, J. Amer. Chem. Soc., 83, 1 102 (1961). (b) The electron pair
in 12 is shown in the equatorial rather than in the axial position because the axial positions are pre-
ferred by the more electronegative groups and the equatorial by the more electropositive groups.
(c) The structure shown in 11 arises from attack of the nucleophile on the face of the tetrahedron
opposite to the apex occupied by the leaving group. We have already discussed the simple bonding
model for 11 in Section 4.2 (p. 175) ; it is readily apparent from this model that 11 could be a transi-
tion state for nucleophilic substitution on any sp3 hybridized atom. The structures 12 and 13, on the
other hand, arise from attack of the nucleophile on an edge of the tetrahedron. If the central atom is
carbon or some other element that does not have empty d orbitals, then there is no simple bonding
model that corresponds to 12 or 13; if d orbitals are present, oriented toward the edge of the tetra-
hedron being attacked, they can accept the electrons of the nucleophile.
See note 63, p. 197 and 66, p. 198.