Page 213 - Mechanism and Theory in Organic Chemistry
P. 213
the rate-determining step, one would expect that the rates of reaction would
decrease in the order I > Br > C1.7Wiuffarin and co-workers interpret their
results as evidence for a reaction path in which the formation of the intermediate
is rate-determining.
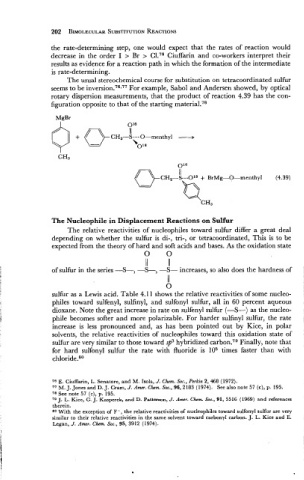
The usual stereochemical course for substitution on tetracoordinated sulfur
seems to be in~ersion.~~.~~ For example, Sabol and Andersen showed, by optical
rotary dispersion measurements, that the product of reaction 4.39 has the con-
figuration opposite to that of the starting material.78
The Nucleophile in Displacement Reactions on Sulfur
The relative reactivities of nucleophiles toward sulfur differ a great deal
depending on whether the sulfur is di-, tri-, or tetracoordinated, This is to be
expected from the theory of hard and soft acids and bases. As the oxidation state
0 0
II II
of sulfur in the series -S-, -S-, -S- increases, so also does the hardness of
II
0
sulfur as a Lewis acid. Table 4.11 shows the relative reactivities of some nucleo-
philes toward sulfenyl, sulfinyl, and sulfonyl sulfur, all in 60 percent aqueous
dioxane. Note the great increase in rate on sulfenyl sulfur (-S-) as the nucleo-
phile becomes softer and more polarizable. For harder sulfinyl sulfur, the rate
increase is less pronounced and, as has been pointed out by Kice, in polar
solvents, the relative reactivities of nucleephiles toward this oxidation state of
sulfur are very similar to those toward sp3 hybridized carbon.79 Finally, note that
for hard sulfonyl sulfur the rate with fluoride is lo5 times faster than with
chloride.80
78 E. Ciuffarin. L. Senatore. and M. Isola. J. Chem. Soc.. Perkin 2, 468 (1972').
.
.
77 M. J. ones ind D. J. Cram, J. Amcr. Cl;em. SOG., 96,2183 (1974). See also note 57 (c), p. 195.
78 See note 57 (c), p. 195.
'8 J. L. Kice, G. J. Kasperek, and D. Patterson, J. Amer. Chem. SOG., 91, 5516 (1969) and references
thirein.
80 With the exception of F-, the relative reactivities of nucleophiles toward sulfonyl sulfur are very
similar to their relative reactivities in the same solvent toward carbonyl carbon. J. L. Kice and E.
Legan, J. Amer. Chem. Soc., 95, 3912 (1974).