Page 218 - Mechanism and Theory in Organic Chemistry
P. 218
Bimolecular Electrophilic Substitution at Saturated Carbon 207
The specific rotation of the initial s-butyl bromide used by Charman,
Hughes, and Ingold was - 15.2". The specific rotation of the product of the
mercury exchange reaction was exactly half that, - 7.6". When mercuric acetate
or mercuric nitrate was used as the cleaving salt, the products showed a specific
rotation of - 7.5" and - 7.8", respectively. Thus this electrophilic substitution
clearly proceeds with retention of configuration.
Stereochemical studies on other mercury exchange reactions have been
carried out, and all point to retention as the predominant pathway.84
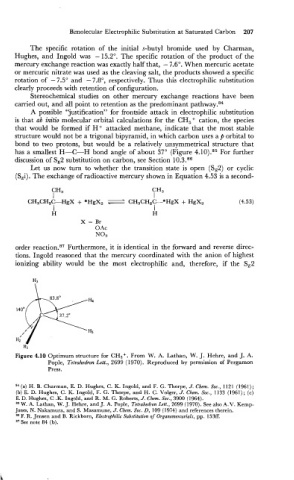
A possible "justification" for frontside attack in electrophilic substitution
is that a6 initio molecular orbital calculations for the CH5+ cation, the species
that would be formed if H+ attacked methane, indicate that the most stable
structure would not be a trigonal bipyramid, in which carbon uses a p orbital to
bond to two protons, but would be a relatively unsymmetrical structure that
has a smallest H-C-H bond angle of about 37" (Figure 4.10).85 For further
discussion of S,2 substitution on carbon, see Section 10.3.86
Let us now turn to whether the transition state is open (SE2) or cyclic
(SEi). The exchange of radioactive mercury shown in Equation 4.53 is a second-
X = Br
OAc
NO3
order reaction.87 Furthermore, it is identical in the forward and reverse direc-
tions. Ingold reasoned that the mercury coordinated with the anion of highest
ionizing ability would be the most electrophilic and, therefore, if the S,2
Figure 4.10 Optimum structure for CHS+. From W. A. Lathan, W. J. Hehre, and J. A.
Pople, Tetrahedron Lett., 2699 (1970). Reproduced by permission of Pergamon
Press.
84 (a) H. B. Charman, E. D. Hughes, C. K. Ingold, and F. G. Thorpe, J. Chem. Soc., 1121 (1961) ;
(b) E. D. Hughes, C. K. Ingold, F. G. Thorpe, and H. C. Volger, J. Chem. SOG., 1133 (1961); (c)
E. D. Hughes, C .K. Ingold, and R. M. G. Roberts, J. Chem. Sor., 3900 (1964).
85 W. A. Lathan, W. J. Hehre, and J. A. Pople, Tetrahedron Lett., 2699 (1970). See also A.V. Kemp-
Jones, N. Nakamura, and S. Masamune, J. Chem. SOG. D, 109 (1974) and references therein.
" F. R. Jensen and B. Rickborn, Electrophilic Substitution of Organomercurials, pp. 153ff.
See note 84 (b).