Page 221 - Mechanism and Theory in Organic Chemistry
P. 221
PROBLEMS
1. Predict which would be more reactive as a nucleophile in S,2 substitution:
2. Suggest an explanation for the fact that the order of reactivity of the halides
toward n-butyl brosylate in acetone is C1- > Br- > I- when (C,H,),N+ is the cation
of the halide salt but I - > Br - > C1- when Li + is the cation.
3. Suggest a reason for the secondary isotope effect, k,/k, = 1.10 in the reactions:
4. Nucleophiles such as NH20H and NH2NH2, which have an electronegative
atom with one or more pairs of unshared electrons adjacent to the nucleophilic center,
are more reactive than might be expected from the nature of the nucleophilic center, as
can be seen from Table 4.5. Suggest a reason for this increased reactivity (this pheno-
menon is called the a-effect).
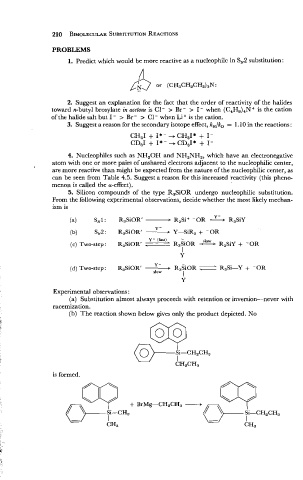
5. Silicon compounds of the type R,SiOR undergo nucleophilic substitution.
From the following experimental observations, decide whether the most likely mechan-
ism is
Y - (fast) -
(c) Two-step: R3SiORt / R3SiOR R3SiY + -OR
I
Y
Y -
+
-OR
R3Si-Y
(d) Two-step: R3SiORr - R~S~OR ;
slow
I
Experimental observations :
(a) Substitution almost always proceeds with retention or inversion-never with
racemization.
(b) The reaction shown below gives only the product depicted. No
is formed.