Page 226 - Mechanism and Theory in Organic Chemistry
P. 226
Kinetics and Stereochemistry 215
kinetic behavior is predicted, and this is usually whatis observed. A sdlkmdy
l<rge added concentraE~kav~ C1-) might lead to a
case
rate depressiin, called a cotnm~n ion efict&was to this cause that Hughes and
Ingold attributed the observed rate decrease for benzhydryl chloride with added
chloride salts.5 Azide ion diverts the carbocation to stable alkyl azide: thfz
amount of azide formed is consistent only_wit_h its formation from an intermedi-
--_
-
-
. - - - - .
he
ate, presumed to be th&R+ ion. which also produces the solvol~i~r~duct.~
-
--
stereochemistry of the benzhydryl reactions is also consistent with the Ingold
mechanism. As we shall see in more detail in Section 5.3, tbae is now abundant
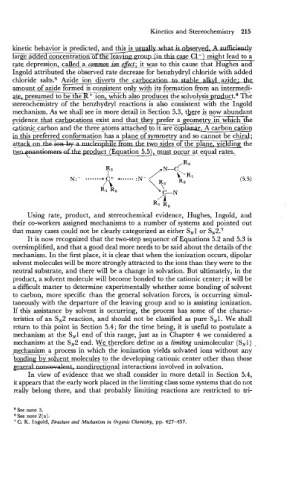
gvdence that carbocations exist and that theygrefer a geometrv in whichthe
-- -
@&on& carbon and the three atoms attached to it are cnplauar. A carbon cation
in this preferred conformation has a plane of symmetry and so cannot beB1;
e
~ a G two sides of the plane. vXding the
-duct t (Equation 5.5), m-occur at_equ_al rates,.
/ R3
R3
Using rate, product, and stereochemical evidence, Hughes, Ingold, and
their co-workers assigned mechanisms to a number of systems and pointed out
that many cases could not be clearly categorized as either S,1 or SN2.7
It is now recognized that the two-step sequence of Equations 5.2 and 5.3 is
oversimplified, and that a good deal more needs to be said about the details of the
mechanism. In the first place, it is clear that when the ionization occurs, dipolar
solvent molecules will be more strongly attracted to the ions than they were to the
neutral substrate, and there will be a change in solvation. But ultimately, in the
product, a solvent molecule will become bonded to the cationic center; it will be
a difficult matter to determine experimentally whether some bonding of solvent
to carbon, more specific than the general solvation forces, is occurring simul-
taneously with the departure of the leaving group and so is assisting ionization.
If this assistance by solvent is occurring, the process has some of the charac-
teristics of an SN2 reaction, and should not be classified as pure SN1. We shall
return to this point in Section 5.4; for the time being, it is useful to postulate a
mechanism at the SN1 end of this range, just as in Chapter 4 we considered a
mechanism at the SN2 end. W~therefore define as a limiting unimolecular (SN1) .
mechanism a process in which the ionization yields solvated ions without any
.--
bondinc by solvent molecules to the developing cationic center other than those
e nmxiircc_ko-n-al interactions involved in solvation.
In view of evidence that we shall consider in more detail in Section 5.4,
it appears that the early work placed in the limiting class some systems that do not
really belong there, and that probably limiting reactions are restricted to tri-
See note 3.
See note 2(a).
C. K. Ingold, Structure and Mechanism in Organic Chemistry, pp. 427-457.