Page 227 - Mechanism and Theory in Organic Chemistry
P. 227
arylmethyl, benzhyd~yl,~ tertiary alkyl, and allylic systems. Even the limiting
S 1 rocess is not without complication, however, because it is possible to show
p.
that, in some instances at least, the ionization process consists of more than one
step.
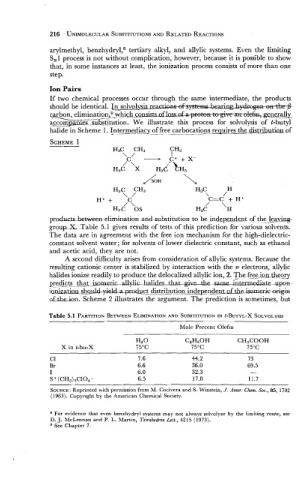
Ion Pairs
If two chemical processes occur through the same intermediate, the products
should be identical. In solvolvsis re--
carbon, elimination which- hsdhpemr- generally
-
\--_--J
accomg;asles substitution. We illustrate this process for solvolysis of t-butyl
halide in Scheme 1. I_n~y_mdjacyffree carbocations requires the distributian~f
\
AH
H3C CH3 H3C H
\ / \ /
C=C + H+
H++ /C\ H3C '
/
H3c 0s H
prdxts-bekween elimination and substitution to be hdqmdent of the leavizg-
group-X Table 5.1 gives results of tests of this prediction for various solvents.
The data are in agreement with the free ion mechanism for the high-dielectric-
constant solvent water; for solvents of lower dielectric constant, such as ethanol
and acetic acid, they are not.
A second difficulty arises from consideration of allylic systems. Because the
resulting cationic center is stabilized by interaction with the .rr electrons, allylic
halides ionize readily to produce the delocalized allylic ion, 2. The - f~ee ion theq
pyedFct_s_that i p hali- the -e -upon-
.. .
1- sh- ct distribution independent of thckmmic origin
ofn. Scheme 2 illustrates the argument. The prediction is sometimes, but
Table 5.1 PARTITION BETWEEN ELIMINATION AND SUBSTITUTION I-BUTYL-X SOLVOLYSIS
IN
Mole Percent Olefin
SOURCE: Reprinted with permission from M. Cocivera and S. Winstein, J. Amer. Chem. Soc., 85, 1702
(1963). Copyright by the American Chemical Society.
For evidence that even benzhydryl systems may not always solvolyze by the limiting route, see
D. J. McLennan and P. L. Martin, Tetrahedron Lett., 4215 (1973).
See Chapter 7.