Page 222 - Mechanism and Theory in Organic Chemistry
P. 222
References for Problems 21 1
0
I I
(c) Acyloxysilanes (leaving group Rf-C-0) are considerably more reactive than
alkoxysilanes.
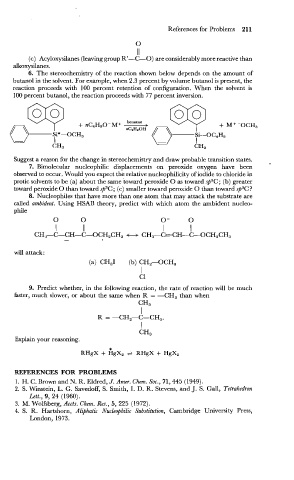
6. The stereochemistry of the reaction shown below depends on the amount of
butanol in the solvent. For example, when 2.3 percent by volume butanol is present, the
reaction proceeds with 100 percent retention of configuration. When the solvent is
100 percent butanol, the reaction proceeds with 77 percent inversion.
Suggest a reason for the change in stereochemistry and draw probable transition states. ,
7. Bimolecular nucleophilic displacements on peroxide oxygen have been
observed to occur. Would you expect the relative nucleophilicity of iodide to chloride in
protic solvents to be (a) about the same toward peroxide 0 as toward sp3C; (b) greater
toward peroxide 0 than toward sp3C; (c,) smaller toward peroxide 0 than toward sp3C?
8. Nucleophiles that have more than one atom that may attack the substrate are
called arnbident. Using HSAB theory, predict with which atom the ambident nucleo-
phile
0 0 0- 0
will attack:
(a) CH31 (b) CH2-OCH,
I
C1
9. Predict whether, in the following reaction, the rate of reaction will be much
faster, much slower, or about the same when R = -CH3 than when
CH3
I
R = -CH,-C-CH3.
I
CH3
Explain your reasoning.
*
RHgX + HgXz + RHgX + HgXz
REFERENCES FOR PROBLEMS
1. H. C. Brown and N. R. Eldred, J. Arner. Chern. Soc., 71,445 (1949).
2. S. Winstein, L. G. Savedoff, S. Smith, I. D. R. Stevens, and J. S. Gall, Tetrahedron
Lett., 9, 24 (1960).
3. M. Wolfsberg, Accts. Chern. Res., 5, 225 (1972).
4. S. R. Hartshorn, Aliphatic Nucleophilic Substitution, Cambridge University Press,
London, 1973.