Page 219 - Mechanism and Theory in Organic Chemistry
P. 219
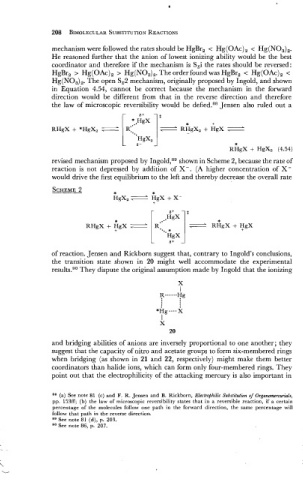
mechanism were followed the rates should be HgBr, < Hg(OAc), < Hg(NO,),.
He reasoned further that the anion of lowest ionizing ability would be the best
coordinator and therefore if the mechanism is SEi the rates should be reversed:
HgBr, > Hg(OAc), > Hg(N0,) ,. The order found was HgBr, < Hg(OAc), <
Hg(NO,),. The open SE2 mechanism, originally proposed by Ingold, and shown
in Equation 4.54, cannot be correct because the mechanism in the forward
direction would be different from that in the reverse direction and therefore
the law of microscopic reversibility would be defied.88 Jensen also ruled out a
a+ t
* .HgX
RHgX + *HgX2 - I.::: ] = .kg,, + =
HgX2
6- *
RHgX + HgXz (4.54)
revised mechanism proposed by Ing~ld,~~ shown in Scheme 2, because the rate of
reaction is not depressed by addition of X-. (A higher concentration of X-
would drive the first equilibrium to the left and thereby decrease the overall rate
SCHEME 2 * *
HgX2 HgX + X-
+
of reaction. Jensen and Rickborn suggest that, contrary to Ingold's conclusions,
the transition state shown in 20 might well accommodate the experimental
results.g0 They dispute the original assumption made by Ingold that the ionizing
and bridging abilities of anions are inversely proportional to one another; they
suggest that the capacity of nitro and acetate groups to form six-membered rings
when bridging (as shown in 21 and 22, respectively) might make them better
coordinators than halide ions, which can form only four-membered rings. They
point out that the electrophilicity of the attacking mercury is also important in
(a) See note 81 (c) and F. R. Jensen and B. Rickborn, Electrophilu Substitution of Organomercurials,
pp. 153ff; (b) the law of microscopic reversibility states that in a reversible reaction, if a certain
percentage of the molecules follow one path in the forward direction, the same percentage will
follow that path in the reverse direction.
See note 81 (d), p. 203.
See note 86, p. 207.