Page 214 - Mechanism and Theory in Organic Chemistry
P. 214
Bimolecular Electrophilic Substitution at Saturated Carbon 203
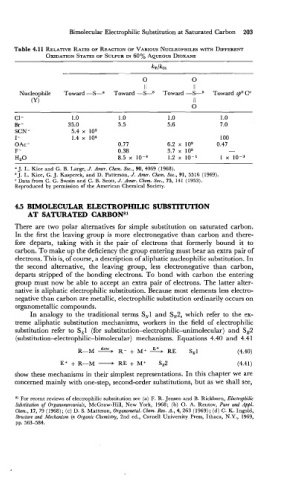
Table 4.11 RELATIVE RATES OF REACTION VARIOUS NUCLEOPHILES WITH DIFFERENT
OF
OXIDATION IN
STATES OF SULFUR 60% AQUEOUS DIOXANE
ll Il
Nucleophile Toward -S-" Toward -SAb Toward -S-b Toward sp3 CC
a J. L. Kice and G. B. Large, J. Amer. Chem. Soc., 90, 4069 (1968).
J. L. Kice, G. J. Kasperek, and D. Patterson, J. Amer. Chem. Soc., 91, 5516 (1969).
Data from C. G. Swain and C. B. Scott, J.'Amer. Chem. Soc., 75, 141 (1953).
Reproduced by permission of the American Chemical Society.
4.5 BIMOLECULAR ELECTROPHILIC SUBSTITUTION
AT SATURATED CARBONB1
There are two polar alternatives for simple substitution on saturated carbon.
In the first the leaving group is more electronegative than carbon and there-
fore departs, taking with it the pair of electrons that formerly bound it to
carbon. To make up the deficiency the group entering must bear an extra pair of
electrons. This is, of course, a description of aliphatic nucleophilic substitution. In
the second alternative, the leaving group, less electronegative than carbon,
departs stripped of the bonding electrons. To bond with carbon the entering
group must now be able to accept an extra pair of electrons. The latter alter-
native is aliphatic electrophilic substitution. Because most elements less electro-
negative than carbon are metallic, electrophilic substitution ordinarily occurs on
organometallic compounds.
In analogy to the traditional terms S,1 and SN2, which refer to the ex-
treme aliphatic substitution mechanisms, workers in the field of electrophilic
substitution refer to S,1 (for substitution-electrophilic-unimolecular) and SE2
(substitution-electrophilic-bimolecular) mechanisms. Equations 4.40 and 4.41
R-M R- + M+ RE SE1 (4.40)
E+ + R-M - + M+ SE~ (4.41)
RE
show these mechanisms in their simplest representations. In this chapter we are
concerned mainly with one-step, second-order substitutions, but as we shall see,
For recent reviews of electrophilic substitution see (a) F. R. Jensen and B. Rickborn, Electrophilic
Substitution of Organornercurials, McGraw-Hill, New York, 1968; (b) 0. A. Reutov, Pure and Appl.
Chem., 17, 79 (1968); (c) D. S. Matteson, Organometal. Chem. Rev. A., 4,263 (1969); (d) C. K. Ingold,
Structure and Mechanism +-I Organic Chemistry, 2nd ed., Cornell University Press, Ithaca, N.Y., 1969,
pp. 563-584.