Page 211 - Mechanism and Theory in Organic Chemistry
P. 211
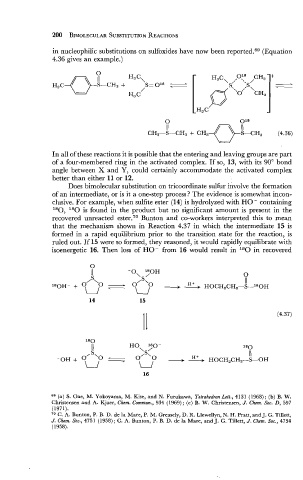
in nucleophilic substitutions on sulfoxides have now been reported.69 (Equation
4.36 gives an example.)
In all of these reactions it is possible that the entering and leaving groups are part
of a four-membered ring in the activated complex. If so, 13, with its 90' bond
angle between X and Y, could certainly accommodate the activated complex
better than either 11 or 12.
Does bimolecular substitution on tricoordinate sulfur involve the formation
of an intermediate, or is it a one-step process? The evidence is somewhat incon-
clusive. For example, when sulfite ester (14) is hydrolyzed with HO - containing
180, 180 is found in the product but no significant amount is present in the
recovered unreacted ester.70 Bunton and co-workers interpreted this to mean
that the mechanism shown in Reaction 4.37 in which the intermediate 15 is
formed in a rapid equilibrium prior to the transition state for the reaction, is
ruled out. If 15 were so formed, they reasoned, it would rapidly equilibrate with
isoenergetic 16. Then loss of HO- from 16 would result in 180 in recovered
69 (a) S. Oae, M. Yokoyama, M. Kise, and N. Furukawa, Tetrahedron Lett., 4131 (1968); (b) B. W.
Christensen and A. Kjaer, Chem. Commun., 934 (1969); (c) B. W. Christensen, J. Chem. Soc. D, 597
(1971).
70 C. A. Bunton, P. B. D. de la Mare, P. M. Greasely, D. R. Llewellyn, N. H. Pratt, and J. G. Tillett,
J. Chem. Soc., 4751 (1958); C. A. Bunton, P. B. D. de la Mare, and J. G. Tillett, J. Chem. Soc., 4754
(1958).