Page 208 - Mechanism and Theory in Organic Chemistry
P. 208
Bimolecular Nucleophilic Substitution at Sulfur 197
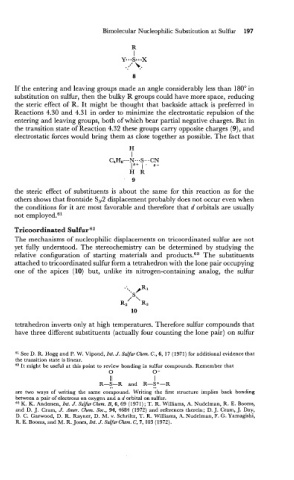
If the entering and leaving groups made an angle considerably less than 180" in
substitution on sulfur, then the bulky R groups could have more space, reducing
the steric effect of R. It might be thought that backside attack is preferred in
Reactions 4.30 and 4.31 in order to minimize the electrostatic repulsion of the
entering and leaving groups, both of which bear partial negative charges. But in
the transition state of Reaction 4.32 these groups carry opposite charges (9), and
electrostatic forces would bring them as close together as possible. The fact that
the steric effect of substituents is about the same for this reaction as for the
others shows that frontside S,2 displacement probably does not occur even when
the conditions for it are most favorable and therefore that d orbitals are usually
not employed.61
Tricoordinated Sulfur 62
The mechanisms of nucleophilic displacements on tricoordinated sulfur are not
yet fully understood. The stereochemistry can be determined by studying the
relative configuration of starting materials and products.63 The substituents
attached to tricoordinated sulfur form a tetrahedron with the lone pair occupying
one of the apices (10) but, unlike its nitrogen-containing analog, the sulfur
tetrahedron inverts only at high temperatures. Therefore sulfur compounds that
have three different substituents (actually four counting the lone pair) on sulfur
See D. R. Hogg and P. W. Vipond, Int. J. Sulfur Chem. C., 6, 17 (1971) for additional evidence that
the transition state is linear.
62 It might be useful at this point to review bonding in sulfur compounds. Remember that
0 0-
II I
R-S-R and R-S+-R
are two ways of writing the same compound. Writing the first structure implies back bonding
between a pair of electrons on oxygen and a d orbital on sulfur.
K. K. Andersen, Int. J. Sulfur Chem. B, 6, 69 (1971); T. R. Williams, A. Nudelman, R. E. Booms,
and D. J. Cram, J. Amer. Chem. Soc., 94, 4684 (1972) and references therein; D. J. Cram, J. Day,
D. C. Garwood, D. R. Rayner, D. M. v. Schriltz, T. R. Williams, A. Nudelman, F. G. Yamagishi,
R. E. Booms, and M. R. Jones, Int. J. Sulfur Chem. C, 7, 103 (1972).