Page 204 - Mechanism and Theory in Organic Chemistry
P. 204
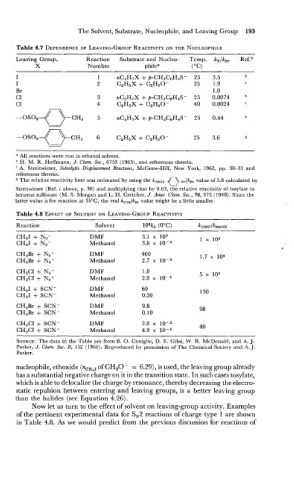
The Solvent, Substrate, Nucleophile, and Leaving Group 193
Table 4.7 DEPENDENCE LEAVING-GROUP REACTIVITY THE NUCLEOPHILE
ON
OF
Leaving Group, Reaction Substrate and Nucleo- Temp. k,/k,, 'Ref.b
X Number philea ("C)
a All reactions were run in ethanol solvent.
H. M. R. Hoffmann, J. Chem. Soc., 6753 (1965), and references therein.
A. Streitwieser, Solvolytic Displacement Reactions, McCraw-Hill, New York, 1962, pp. 30-31 and
references therein.
The relative reactivity here was estimated by using the koso, 0- .,/kB, value of 5.8 calculated by
\=/
Streitwieser (Ref. c above, p. 30) and multiplying that by 0.63, the relative reactivity of tosylate to
benzene sulfonate (M. S. Morgan and L. H. Cretcher, J. Amer. Chem. Soc., 70, 375 (1948). Since the
latter value is for reaction at 35'C, the real koT,/kB, value might be a little smaller.
Table 4.8 EFFECT OF SOLVENT LEAVING-GROUP REACTIVITY
ON
Reaction Solvent 104kz (OOC) k ~ ~ ~ / k ~ e ~ ~
CH~I + N~ - DMF 3.1 103 1 x 105
CH31 + N3- Methanol 3.0 x
CH,Br + N3- DMF 460 1.7 104
CH3Br + N3- Methanol 2.7 x
CH3CI + N3- DMF I .O 5 x 103
CH3CI + N3- Methanol 2.0 x 10-4
CH31 + SCN- DMF 69 130
CH31 + SCN- Methanol 0.30
CH3Br + SCN- DMF 9.8 98
CH3Br + SCN- Methanol 0.10
CH3Cl + SCN- DMF 2.0 x lo-z 40
CH3Cl + SCN- Methanol 4.9 x
SOURCE: The data in the Table are from B. 0. Coniglio, D. E. Giles, W. R. McDonald, and A. J.
Parker, J. Chem. Soc. B, 152 (1966). Reproduced by permission of The Chemical Society and A. J.
Parker.
nucleophile, ethoxide (nCH3, of CH30- = 6.29), is used, the leaving group already
has a substantial negative charge on it in the transition state. In such cases tosylate,
which is able to delocalize the charge by resonance, thereby decreasing the electro-
static repulsion between entering and leaving groups, is a better leaving group
than the halides (see Equation 4.26).
Now let us turn to the effect of solvent on leaving-group activity. Examples
of the pertinent experimental data for S,2 reactions of charge type 1 are shown
in Table 4.8. As we would predict from the previous discussion for reactions of