Page 217 - Mechanism and Theory in Organic Chemistry
P. 217
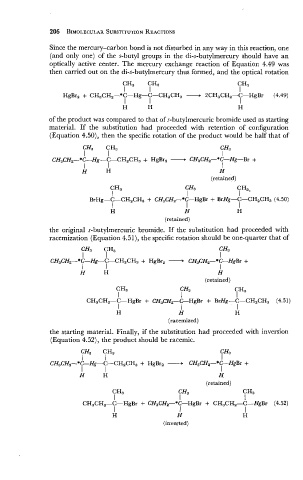
Since the mercury-carbon bond is not disturbed in any way in this reaction, one
(and only one) of the s-butyl groups in the di-s-butylmercury should have an
optically active center. The mercury exchange reaction of Equation 4.49 was
then carried out on the di-s-butylmercury thus formed, and the optical rotation
of the product was compared to that of s-butylmercuric bromide used as starting
material. If the substitution had proceeded with retention of configuration
(Equation 4.50), then the specific rotation of the product would be half that of
CH3 CH3 cH3
I I I
CH3CH2-*C-Hg-C-CH2CH3 + HgBr, - CH3CH2-*C-Hg-Br +
I I I
H H H
(retained)
CH3 cH3 CH3.
I I I
BrHg-C-CH2CH3 + CH3CH2-*C-HgBr + BrHg-C-CH2CH3 (4.50)
I I I
H H H
(retained)
the original s-butylmercuric bromide. If the substitution had proceeded with
racemization (Equation 4.51), the specific rotation should be one-quarter that of
CH3 CH, cH3
I I I
CH3CH2-*C-Hg-C-CH2CH3 + HgBr, - CH3CH2--*C-HgBr +
I I I
H H H
(retained)
CH3 cH3 CH3
I 1 I
CH3CH2-C-HgBr + CH3CH2-C-HgBr + BrHg-C-CH2CH3 (4.51)
I I I
H H
(racemized)
the starting material. Finally, if the substitution had proceeded with inversion
(Equation 4.52), the product should be racemic.
CH,CH,-*c-H~-C-CH,CH, + HgBr2 ---+ CH,CH,-*C--H~B~ +
I I I
H H Ei:
(retained)
CH3 cH3 CH3
I I I
CH3CH2-C-HgBr + CH3CH2-*C-HgBr + CH,CH,-C-HgBr (4.52)
I I I
H
(inverted)