Page 228 - Mechanism and Theory in Organic Chemistry
P. 228
Kinetics and Stereochemistry 217
not always, verified in practice; there is a marked tendency in many systems to
favor the unrearranged product.1°
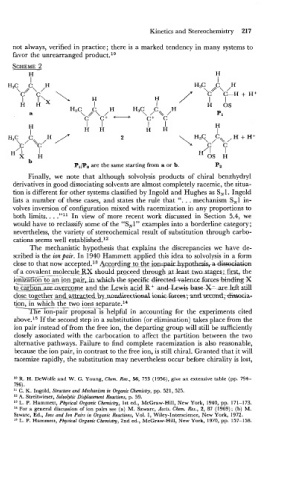
I I
H3C C H
\c/ \ / C-H + Hi
I F\, \ H
H H 1 H 0s
\<\/
C H H3C C H
a PI
C C+ C c
I I 1 I H
H H H H
b
P1/P, are the same starting from a or b. pz
Finally, we note that although solvolysis products of chiral benzhydryl
derivatives in good dissociating solvents are almost completely racemic, the situa-
tion is different for other systems classified by Ingold and Hughes as SN1. Ingold
lists a number of these cases, and states the rule that ". . . mechanism SN1 in-
volves inversion of configuration mixed with racemization in any proportions to
both limits. . . ."ll In view of more recent work discussed in Section 5.4, we
would have to reclassify some of the "S,1" examples into a borderline category;
nevertheless, the variety of stereochemical result of substitution through carbo-
cations seems well established.12
The mechanistic hypothesis that explains the discrepancies we have de-
scribed is the ion pair. In 1940 Hammett applied this idea to solvolysis in a form
close to that now accepted.13 Ac~r&n~tflhexqadqp~-ad~n
of a covalent molecul.e~RX should pro~ceed through at least ~wastagm;. first, the
-----.- - .-
ionlzatlon to-~ io_apai~+in.whi&t-he specific direct&--valence forces &&kg X
to carbon arenvs~o-m-e and .the Lewis., acid. R + .and-L&- base- Xz-are .left still
close together and at~~ed-.by~.m~rectional ion&-fsF~s ;-and -second,- dimcia-
tion, in which .- the two ..~. ions separate.14
..
--The ion-pair proposal is helpful in accounting for the experiments cited
above.15 If the second step in a substitution (or elimination) takes place from the
ion pair instead of from the free ion, the departing group will still be sufficiently
closely associated with the carbocation to affect the partition between the two
alternative pathways. Failure to find complete racemization is also reasonable,
because the ion pair, in contrast to the free ion, is still chiral. Granted that it will
racemize rapidly, the substitution may nevertheless occur before chirality is lost,
lo R. H. DeWolfe and W. G. Young, Chem. Rev., 56, 753 (1956), give an extensive table (pp. 794-
796).
l1 C. K. Ingold, Structure and Mechanism in Organic Chemistry, pp. 521, 525.
l2 A. Streitwieser, Solvolytic Displacement Reactions, p. 59.
l3 L. P. Harnrnett, Physical Organic Chemistry, 1st ed., McGraw-Hill, New York, 1940, pp. 171-173.
l4 For a general discussion of ion pairs see (a) M. Szwarc, Accts. Chem. Res., 2, 87 (1969); (b) M.
Szwarc, Ed., Ions and Ion Pairs in Organic Reactions, Vol. I, Wiley-Interscience, New York, 1972.
l5 L. P. Hamrnett, Physical Organic Chemistry, 2nd ed., McGraw-Hill, New York, 1970, pp. 157-158.