Page 197 - Mechanism and Theory in Organic Chemistry
P. 197
SN2 reaction in a protic solvent can be predicted quantitatively if the n value of
the attacking reagent and a second parameter, s, which represents the sensitivity
of the substrate to the reagent's nucleophilicity, are known.41 The quantitative
relationship between these two parameters and the rate is the linear free-energy
relationship (see Section 2.2)
k
log - = ns
ko
in which k is the rate of an SN2 reaction in which the nucleophile has nucleo-
philicity n and the substrate has sensitivity s. The constant k, is the rate of the
standard reaction to which all others are compared. Swain and Scott have chosen
the reaction of methyl bromide with water at 25OC as that reaction. The sub-
strate, methyl bromide, is arbitrarily assigned an s value of 1.
The parameter n for a given nucleophile, Y, then is defined by the following
equation :
+
Y
n = log ~CHSB~
k~~Q~r+
H~O
To determine n directly for a specific nucleophile, its rate of reaction with methyl
bromide is measured at 25OC; the log of the ratio of this rate to the rate of reac-
tion of the same substrate with H,O gives n. If Y is a better nucleophile than
water, n will be positive; if Y is a worse nucleophile, n will be negative. Water
itself has an n value of zero. In Table 4.4 are listed the majority of the n values
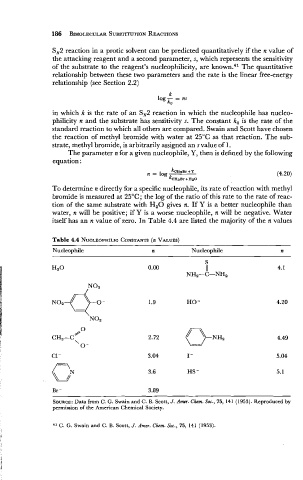
Table 4.4 NUCLEOPHILIC CONSTANTS VALUES)
(n
Nucleophile n Nucleophile n
1.9 HO-
SOURCE: Data from C. G. Swain and C. B. Scott, J. Amr. Chm. Soc., 75, 141 (1953). Reproduced by
permission of the American Chemical Society.
41 C. G. Swain and C. B. Scott, J. Amer. Chem. Soc., 75, 141 (1953).