Page 196 - Mechanism and Theory in Organic Chemistry
P. 196
The Solvent, Substrate, Nucleophile, and Leaving Group 185
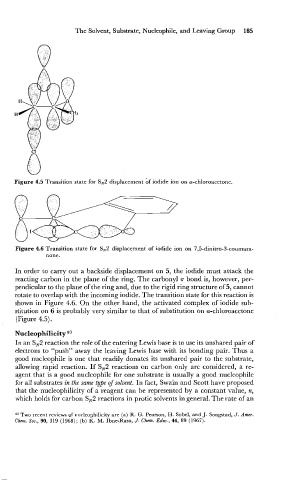
Figure 4.5 Transition state for SN2 displacement bf iodide ion on a-chloroacetone.
Figure 4.6 Transition state for SN2 displacement of iodide ion on 7,5-dinitro-3-coumara-
none.
In order to carry out a backside displacement on 5, the iodide must attack the
reacting carbon in the plane of the ring. The carbonyl rr bond is, however, per-
pendicular to the plane of the ring and, due to the rigid ring structure of 5, cannot
rotate to overlap with the incoming iodide. The transition state for this reaction is
shown in Figure 4.6. On the other hand, the activated complex of iodide sub-
stitution on 6 is probably very similar to that of substitution on a-chloroacetone
(Figure 4.5).
Nucleophilicity 40
In an SN2 reaction the role of the entering Lewis base is to use its unshared pair of
electrons to "push" away the leaving Lewis base with its bonding pair. Thus a
good nucleophile is one that readily donates its unshared pair to the substrate,
allowing rapid reaction. If SN2 reactions on carbon only are considered, a re-
agent that is a good nucleophile for one substrate is usually a good nucleophile
for all substrates in the same type of solvent. In fact, Swain and Scott have proposed
that the nucleophilicity of a reagent can be represented by a constant value, n,
which holds for carbon SN2 reactions in protic solvents in general. The rate of an
40 TWO recent reviews of nucleophilicity are (a) R. G. Pearson, H. Sobel, and J. Songstad, J. Amer.
Chem. Soc., 90, 319 (1968); (b) K. M. Ibne-Rasa, J. Chem. Educ., 44, 89 (1967).