Page 189 - Mechanism and Theory in Organic Chemistry
P. 189
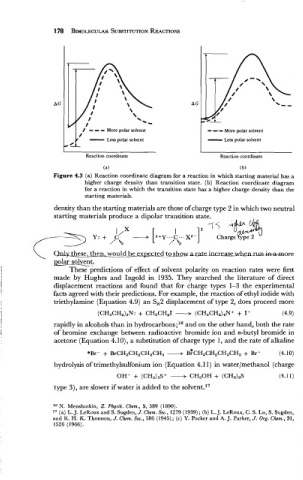
/ - - - More polar solvent - - - More polar solvent
/
Less
6 - polar solvent - polar solvent
Less
0-
L
Reaction coordinate Reaction coordinate
(a) (b)
Figure 4.3 (a) Reaction coordinate diagram for a reaction in which starting material has a
higher charge density than transition state. (b) Reaction coordinate diagram
for a reaction in which the transition state has a higher charge density than the
starting materials.
density than the starting materials are those of charge type 2 in which two neutral
starting materials produce a dipolar transition state.
\ Qnly these, then, wou Id bbe~xp_ec:fcxd toshaw arakincreas~_uvhenr~~1-iRaaaore
\
solcrt:
These predictions of effect of solvent polarity on reaction rates were first
made by ~;~hes and Ingold in 1935. ~ h k ~
searched the literature of direct
displacement reactions and found that for charge types 1-3 the experimental
facts agreed with their predictions. For example, the reaction of ethyl iodide with
triethylamine (Equation 4.9) an S,2 displacement of type 2, does proceed more
(CH3CH2)3N: + CH3CH,I + (CH3CH2)4N+ + I- (4.9)
rapidly in alcohols than in hydrocarbons;16 and on the other hand, both the rate
of bromine exchange between radioactive bromide ion and n-butyl bromide in
acetone (Equation 4.10), a substitution of charge type 1, and the rate of alkaline
hydrolysis of trimethylsulfonium ion (Equation 4.1 1) in waterlmethanol (charge
OH- + (CH3)3S+ + CH30H + (CH3)2S (4.1 1)
type 3), are slower if water is added to the solvent."
l6 N. Menshutkin, Z. Physik. Chem., 5, 589 (1890).
l7 (a) L. J. LeRoux and S. Sugden, J. Chem. Soc., 1279 (1939) ; (b) L. J. LeRoux, C. S. Lu, S. Sugden,
and R. H. K. Thomson, J. Chem. Soc., 586 (1945); (c) Y. Pocker and A. J. Parker, J. Org. Chem., 31,
1526 (1966).