Page 186 - Mechanism and Theory in Organic Chemistry
P. 186
Stereochemistry of the S,2 Reaction 175
using three sp2 orbitals for bonding with the nonreacting ligands and the remain-
ing p orbital for forming partial bonds with X and Y. The geometry is that of a
trigonal bipyramid with the entering and leaving groups in the apical positions.
Because of its accordance with experimental facts, and because of its compati-
bility with our ideas about bonding, this transition state has been universally
accepted. See Section 10.3 for a further discussion of the SN2 transition state.
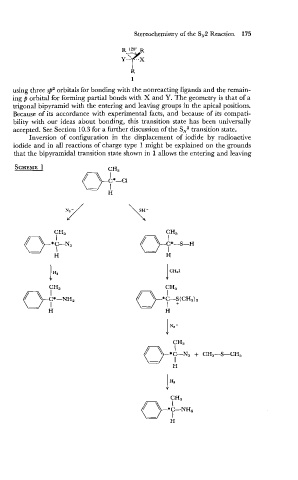
Inversion of configuration in the displacement of iodide by radioactive
iodide and in all reactions of charge type 1 might be explained on the grounds
that the bipyramidal transition state shown in 1 allows the entering and leaving