Page 395 - Mechanism and Theory in Organic Chemistry
P. 395
trioxide in organic s01vents.l~~ When SO, is used in fairly dilute solution, the
attacking species is SO, itself. In concentrated sulfuric acid, however, the
mechanism is more complex. Fuming sulfuric acid (in which the mole fraction of
SO, > 0.5) is actually a mixture of SO, and ionized and nonionized monomers,
dimers, trimers, and tetramers of H2S04 (the three latter formed by dehydration).
As more water is added, the tetramer and trimer disappear, and the amount of
dimer decreases. The reactive species in sulfuric acid thus depends on the amount
of water in the acid and on the reactivity of the substrate. The reactive species in
aqueous sulfuric acid are H2S04 + and H2S207, the latter being more important
at higher acid concentrations. In fuming sulfuric acid H3S207+ and H2S4013
are also inv01ved.l~~
Aromatic compounds are usually readily alkylated or acylated by a Friedel-
Crafts reaction.150 The combination of reagents used most commonly for aro-
matic alkylation is an alkyl halide with a strong Lewis acid (Equation 7.65).
However, alkenes, alcohols, mercaptans, and a number of other types of organic
R-X + A + ArH + Ar-R + AX + H+ (7.65)
compounds also alkylate aromatic rings when a Friedel-Crafts catalyst is present.
The order of reactivity of Lewis acids as catalysts varies from reaction to reaction
but is most commonly AlC1, > SbC1, > FeC1, > TiC1, > SnC1, > TiC1, >
TeC1, > BiC1, > ZnC1,. The attacking species is sometimes the carbocation
- -
6 + 6 -
itself and sometimes an alkyl halide-Lewis acid complex (e.g., R-X..-AlC1,).
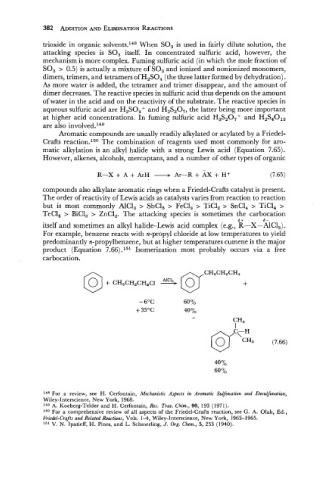
For example, benzene reacts with n-propyl chloride at low temperatures to yield
predominantly n-propylbenzene, but at higher temperatures cumene is the major
product (Equation 7.66).151 Isomerization most probably occurs via a free
carbocation.
148 For a review, see H. Cerfontain, Mechanistic Asjects in Aromatic Sulfonation and Desulfonation,
Wiley-Interscience, New York, 1968.
140 A. Koeberg-Telder and H. Cerfontain, Rec. Trau. Chim., 90, 193 (1971).
150 For a comprehensive review of all aspects of the Friedel-Crafts reaction, see G. A. Olah, Ed.,
Friedel-Crafts and Related Reactions, Vols. 1-4, Wiley-Interscience, New York, 1963-1965.
151 V. N. Ipatieff, H. Pines, and L. Schmerling, J. Org. Chem., 5, 253 (1940).