Page 399 - Mechanism and Theory in Organic Chemistry
P. 399
Studies of the effect of base concentration on rate also provide strong sup-
port for the two-step mechanism. The simple displacement mechanism with
transition state 65 should be first-order in base, as can be seen from the rate
equation for this mechanism,
rate = k,[Ar][E+][B] (7.74)
In the two-step mechanism, if k,[B]/k-, >> 1, no base catalysis whatsoever
should be observed ; if k,p] /k-, << 1, a linear dependence on base is expected;
and if k,[B]/k-, % 1, nonlinear dependence on base should result.
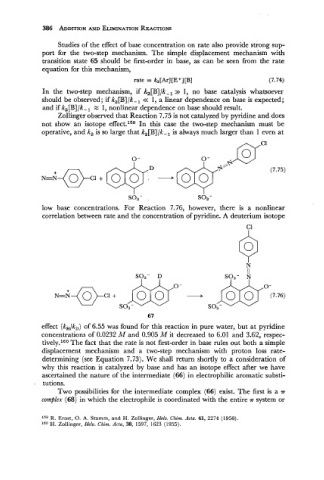
Zollinger observed that Reaction 7.75 is not catalyzed by pyridine and does
not show an isotope effect.159 In this case the two-step mechanism must be
operative, and k, is so large that k,[B]/k-, is always much larger than 1 even at
low base concentrations. For Reaction 7.76, however, there is a nonlinear
correlation between rate and the concentration of pyridine. A deuterium isotope
C1
effect (k,/k,) of 6.55 was found for this reaction in pure water, but at pyridine
concentrations of 0.0232 M and 0.905 M it decreased to 6.01 and 3.62, respec-
tively.160 The fact that the rate is not first-order in base rules out both a simple
displacement mechanism and a two-step mechanism with proton loss rate-
determining (see Equation 7.73). We shall return shortly to a consideration of
why this reaction is catalyzed by base and has an isotope effect after we have
ascertained the nature of the intermediate (66) in electrophilic aromatic substi-
tutions.
Two possibilities for the intermediate complex (66) exist. The first is a rr
complex (68) in which the electrophile is coordinated with the entire rr system or
159 R. Ernst, 0. A. Stamm, and H. Zollinger, Helv. Chim. Acta. 41, 2274 (1958).
160 H. Zollinger, Helv. Chim. Acta, 38, 1597, 1623 (1955).